Abstract
Acute kidney injury (AKI) can develop after multiple wasp or bee stings. The etiology is the acute tubular necrosis secondary to shock, pigment toxicity, interstitial nephritis, or direct nephrotoxicity of venom. We report a 40-year-old female who presented with oliguric AKI after a single wasp sting on her hand. Her history, examination, and investigations did not support any of the established causes of AKI in such settings. She did not improve with supportive management and dialysis, and kidney biopsy showed acute cortical necrosis (ACN). This is the first report of ACN after a single wasp sting.
INTRODUCTION
Wasps and bees are venomous arthropods belonging to the order Hymenoptera. The order consists of three families: Apidae (bees), Vespidae (wasps), and Formicidae (ants).Citation1 Most wasp or bee sting victims do not seek attention due to the minor, self-limiting, and localized nature of symptoms. Acute kidney injury (AKI) is a rare but important life-threatening complication that forces patients to seek medical care. AKI usually develops after multiple stings. The usual underlying lesion is acute tubular necrosis (ATN), and the course is characterized by complete recovery. We report an unusual case of AKI after a single wasp sting which turned out to be due to acute cortical necrosis (ACN).
CASE HISTORY
A 40-year-old female was accidentally stung by a wasp over her left hand while working in her backyard in a rural hilly area of Himachal Pradesh, India. She noted local pain and mild swelling. She was seen at a local healthcare center and received oral H2 blockers. The pain and swelling decreased, but the urine output progressively declined, she became anuric and developed nausea and vomiting over the next 4 days, at which time she was referred to our hospital. Historically, she did not have any hypotension, respiratory distress, swelling of lips, cutaneous allergic symptoms like wheal, urticaria or pruritus, cola colored urine or alternative medicine intake. On examination, she had anasarca and mild pallor. The pulse rate was 80 beats/min, the respiratory rate was 22/min and the blood pressure 134/82 mm Hg. Local examination was unremarkable and systemic examination revealed bilateral basal rales. Initial investigations revealed severe uremia and hyperkalemia, and she was given urgent hemodialysis.
Hematological investigations revealed the following: a hemoglobin level of 9.8 g/dL, a total leukocyte count of 13,000 cells/mm3 with 72% polymorphonuclear cells and 26% lymphocytes, a platelet count of 72,000 cells/mm3, an erythrocyte sedimentation rate of 40 mm/h, a normocytic and normochromic peripheral blood picture without schistocytes, a corrected reticulocyte count of 0.8%, an undetectable plasma hemoglobin, a prothrombin time of 14 s (control 14 s), an activated partial thromboplastin time of 30 s (control 25–32 s), a serum fibrinogen level of 8.8 g/L, and positive D-dimers. Levels of biochemical parameters were as follows: serum creatinine 8.9 mg/dL, blood urea 165 mg/dL, total proteins 5.7 g/dL, albumin 3.1 g/dL, total bilirubin 0.35 mg/dL, aspartate aminotransferase 278 U/L, alanine aminotransferase 155 U/L, lactate dehydrogenase 856 U/L, alkaline phosphatase 100 U/L, calcium 7.9 mg/dL, and inorganic phosphorus 5.6 mg/dL. Urinalysis showed 1+ dipstick proteinuria, 20–25 RBCs/hpf. There were no pus cells or eosinophils. Urine was negative for myoglobin and hemoglobin. Glucose-6-phosphate dehydrogenase enzyme levels were within normal limits. Ultrasonography showed a kidney size of 12.5 cm on either side.
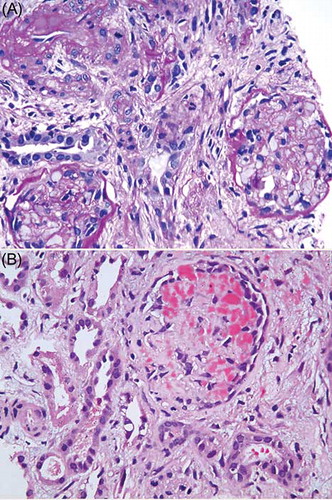
She received alternate-day hemodialysis for 2 weeks, at which time the urine output had improved marginally to 300 mL/day. At this time, a kidney biopsy was done. Light microscopy () showed large areas of cortical necrosis. The viable glomeruli showed fibrillar material with crown of podocytes in some of them. Hemorrhages and edema were present in the interstitium. Arterioles showed subendothelial mucoid material suggestive of subacute thrombotic microangiopathy. The viable tubules showed regenerating tubular necrosis. Immunofluorescence studies were negative for immunoglobulins (IgG, IgM, or IgA), C3, C1q, or light chains (κ or λ). Electron microscopy showed swollen endothelial cells and the presence of subendothelial fluffy material.
Figure 1. Photomicrograph shows (A) glomerular and tubular necrosis and (B) fibrin in the glomerular capillary loops (H&E, ×200). Both panels show interstitial fibrosis.
Over the next 4 weeks, her urine output improved gradually, and she became dialysis independent. At the last follow-up visit after 12 weeks, the serum creatinine had stabilized at 4.3 mg/dL. Urinalysis showed 1+ albumin and 3–4 leukocytes/hpf. A repeat ultrasound examination showed 9.5 and 9.3 cm kidneys with smooth outlines. There was no calcification.
DISCUSSION
Wasp or bee stings are reported from all parts of the world as accidents or after occupational exposure, especially in rural areas. No significant morbidity apart from mild local reaction is seen in an overwhelming majority. Rarely, severe anaphylaxis or the involvement of organ systems may develop. Fatalities after venomous stings are uncommon,Citation2 with severe anaphylaxis rather than toxic effects of venom being the cause of mortality.Citation3 Therefore, all victims should be monitored for local or systemic allergic events after wasp or bee stings. In this regard, detailed guidelines addressing diagnosis, observation, and management including the use of venom immunotherapy have been published.Citation4
Wasp or bees are the most common stinging insects in the hilly areas of North India.Citation5 Systemic manifestations usually develop when the victim is attacked by a swarm of insects and may include hemolysis, rhabdomyolysis, thrombocytopenia, disseminated intravascular coagulation, hepatocellular damage, hypotension, AKI, myocarditis, myocardial infarction, or neurotoxic effects.Citation4,6–9 By contrast, a single sting can lead to anaphylaxis in a previously sensitized individual.
AKI is a well-recognized complication of wasp or bee stings. This form of AKI has been reported, especially from tropical and subtropical areas possibly due to the unique geographical and socioeconomic circumstances. At a tertiary care teaching hospital in Nepal, 11 patients with wasp stings were seen over a 21-month period, 9 of whom had AKI.Citation10,11–13 In a series of five patients with AKI after hornet stings seen over 12 years at our institute, all patients had suffered multiple stings, ranging from 22 to 63.Citation14 In one of the largest series of victims of swarming hornet attacks who reported to a large city hospital in Vietnam, AKI was seen in 38 out of 65 victims, with almost three-fourths requiring renal replacement therapy.Citation6 Patients who died, or who had AKI, or who had shock exhibited higher sting burdens (>50 stings). Delayed hypotension was seen in some patients and was attributed to the possible vasodilatory effects of the large amount of injected venom rather than anaphylaxis.
The mechanism of AKI ranges from tubular injury that is secondary to hemolysis or rhabdomyolysis, hypovolemic or anaphylactic shock, venom-induced acute interstitial nephritis, direct nephrotoxicity of venom, or a combination of the above.Citation11,14–16 This is supported by biopsy evidence in humans and by experimental evidence in rats where vasoconstriction, rhabdomyolysis, and a direct nephrotoxic effect on tubules were seen after exposure to Africanized bee venom.Citation17 Enzymes like phospholipase A2 and hyaluronidase; active peptides like melittin, amines like histamine and serotonin; and others like mastoparan, kinins, acetylcholine, and antigen 5 have been identified as pathogenic agents in the venoms.Citation18–21 In addition to AKI, wasp or bee stings have also been associated with reports of nephrotic syndrome and type 1 renal tubular acidosis.22–26
Our patient had a single wasp sting on her hand and went on to develop AKI without any evidence of hypotension, severe allergic manifestations, hemolysis, or rhabdomyolysis. As the recovery of renal function was delayed and the cause of renal failure was unclear, a renal biopsy was done. Surprisingly, the biopsy revealed ACN with some features suggestive of thrombotic microangiopathy. There is one previous report of AKI secondary to thrombotic microangiopathy and patchy cortical necrosis in a young man with over 50 wasp stings.Citation27 Unlike in our case, the clinical course of this patient was marked by disseminated intravascular coagulation, rhabdomyolysis, hepatic necrosis, and acute respiratory distress syndrome. Our patient showed thrombocytopenia, but there were no fragmented cells. A literature search did not reveal any other report of ACN following insect stings. Therefore, we presume that our patient is the first case where ACN has been seen after a single wasp sting. The possible cause for this is unclear, but a direct effect, where the renal endothelium bore most of the brunt of injury may be responsible.
This case also highlights the need of renal biopsy in a patient where the cause of renal failure is unclear. The need for early diagnosis of allergic interstitial nephritis, so that it can be promptly treated with steroids without delay, also justifies an early renal biopsy in selected atypical cases.Citation15,16 The prognosis for AKI in wasp sting victims is very good.Citation6,8 The recovery was incomplete in our patient in view of the patchy ACN.
In conclusion, ACN can develop rarely even after a single wasp sting. A renal biopsy should be considered if no cause for AKI is apparent or recovery is delayed in wasp sting victims.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Diaz JH, Recognition M. Prevention of hymenopteran stings and allergic reactions in travelers. J Trav Med. 2009;16(5):357–364.
- Langley RL. Animal-related fatalities in the United States—an update. Wilderness Environ Med. 2005;16(2):67–74.
- Bury D, Langlois N, Byard RW. Animal-related ratalities—Part II: characteristic autopsy findings and variable causes of death associated with envenomation, poisoning, anaphylaxis, asphyxiation, and sepsis. J. Forensic Sci. 2012;57(2):375–380.
- Krishna MT, Ewan PW, Diwakar L, . Diagnosis and management of hymenoptera venom allergy: British Society for Allergy and Clinical Immunology (BSACI) guidelines. Clin Exp Allergy. 2011;41(9):1201–1220.
- Goel S, Gupta H, Mazta SR. Epidemiological profile of bite cases admitted at a 50 bedded Community Health Centre of Himachal Pradesh, India. Int J Health. 2008;7(1 ). DOI: 10.5580/1328.
- Xuan BH, Mai HL, Thi TX, Thi MT, Nguyen HN, Rabenou RA. Swarming hornet attacks: shock and acute kidney injury—a large case series from Vietnam. Nephrol Dial Transplant. 2010;25(4):1146–1150.
- Broides A, Maimon M, Landau D, Press J, Lifshitz M. Multiple hymenoptera stings in children: clinical and laboratory manifestations. Eur J Pediatr. 2010;169(10):1227–1231.
- Thiruventhiran T, Goh BL, Leong CL, Cheah PL, Looi LM, Tan SY. Acute renal failure following multiple wasp stings. Nephrol Dial Transplant. 1999;14(1):214–217.
- Vachvanichsanong P, Dissaneewate P. Acute renal failure following wasp sting in children. Eur J Pediatr. 2009;168(8):991–994.
- Paudel B, Paudel K. A study of wasp bites in a tertiary hospital of western Nepal. Nepal Med Coll J. 2009;11(1):52–56.
- Chugh KS, Sharma BK, Singhal PC. Acute renal failure following hornet stings. J Trop Med Hyg. 1976;79(2):42–44.
- Hirachan P, Kharel T, Shah DS, Ball J. Renal replacement therapy in Nepal. Hemodialysis Int. 2010;14(4):383–386.
- Jha V, Chugh KS. Community-acquired acute kidney injury in Asia. Semin Nephrol. 2008;28(4):330–347.
- Sakhuja V, Bhalla A, Pereira BJ, Kapoor MM, Bhusnurmath SR, Chugh KS. Acute renal failure following multiple hornet stings. Nephron. 1988;49(4):319–321.
- Chao YW, Yang AH, Ng YY, Yang WC. Acute interstitial nephritis and pigmented tubulopathy in a patient after wasp stings. Am J Kidney Dis. 2004;43(2):e15–9.
- Sharma A, Wanchu A, Mahesha V, Sakhuja V, Bambery P, Singh S. Acute tubulo-interstitial nephritis leading to acute renal failure following multiple hornet stings. BMC Nephrology. 2006;7(1):1–4.
- Grisotto LS, Mendes GE, Castro I, . Mechanisms of bee venom-induced acute renal failure. Toxicon. 2006;48(1):44–54.
- Hamilton RG. Diagnosis and treatment of allergy to hymenoptera venoms. Curr Opin Allergy Clin Immunol. 2010;10(4):323–329.
- Vetter RS, Ms VPK. Bites and stings of medically important venomous arthropods. Int J Dermatol. 1998;37(7):481–496.
- Incorvaia C, Mauro M, Pravettoni V, Pucci S. Hypersensitivity to Hymenoptera venom: advances in diagnosis and implications for treatment. Recent Pat Inflamm Allergy Drug Discov. 2011;5(2):128–135.
- Gjersoe J, Hundstand S. Venoms: Sources, Toxicity and Therapeutic Uses. New York: Nova Science; 2010.
- Kaarthigeyan K, Sivanandam S, Jothilakshmi K, Matthai J. Nephrotic syndrome following a single bee sting in a child. Indian J Nephrol. 2012;22(1):57–58.
- Cuoghi D, Venturi P, Cheli E. Bee sting and relapse of nephrotic syndrome. Child Nephrol Urol. 1988;9(1–2):82–83.
- D’Cruz S, Chauhan S, Singh R, Sachdev A, Lehl S. Wasp sting associated with type 1 renal tubular acidosis. Nephrol Dial Transplant. 2008 ;23(5):1754–1755.
- Agarwal V, Dcruz S, Sachdev A, Singh R, Kapoor V. Quadriparesis following wasp sting: an unusual reaction. Indian J Med Sci. 2005;59(3):117–119.
- Tasic V. Nephrotic syndrome in a child after a bee sting. Pediatric Nephrology. 2000;15(3–4):245–247.
- George P, Pawar B, Calton N, Mathew P. Wasp sting: an unusual fatal outcome. Saudi J Kidney Dis Transpl. 2008;19(6):969–972.
