Abstract
Hibiscus sabdariffa Linn. (HS) is a tropical wild plant with antioxidant, antibacterial, antihypertensive, and lipid-lowering properties. In several animal models, HS aqueous extracts reduced the severity of the multi-organ injuries such as hypertension and diabetic nephropathy. One of the multiorgan injuries is chronic kidney disease (CKD), which results from the loss of nephron function. HS was used in a 5/6 nephrectomy (5/6 Nx) rat model to determine if it could attenuate the progression of CKD. HS (250 mg/kg/day) or placebo was orally administered to 5/6 Nx male Sprague-Dawley rats. The Nx+HS group had fewer renal injuries as measured by blood urea nitrogen, serum creatinine, creatinine clearance, and renal pathology when compared with the Nx group. In order to determine which property of HS, either vasodilatory and/or antioxidant, was important in attenuating the progression of CKD, systolic blood pressure (SBP) and serum levels of malondialdehyde (MDA) were assessed. In the Nx+HS group, the SBP and the serum levels of MDA were significantly lower at Week 7. In conclusion, through either antihypertensive and/or antioxidant properties, HS was able to attenuate the progression of renal injury after 5/6 Nx. Hence, HS should be considered as one of the new, promising drugs that can be used to attenuate the progression of CKD.
INTRODUCTION
Chronic kidney disease (CKD) is a worldwide health problem that progressively causes the kidney to lose its function; in other words, it is a nonspecific renal injury induced from nephron loss.Citation1,2 Over the past decade, the prevalence of end stage renal disease has increased by more than 30%Citation3 and has significantly affected the global health care systems.Citation4 Physiologic factors (e.g., high blood pressure and anemia) and/or molecular factors (e.g., increases in oxidative stress and transforming growth factor-β1) all contribute to the progressive deterioration of the kidney and functions of other organs in CKD patients. Even though there are many new drugs and techniques in preventing CKD, the number of patients with the disease continues to increase. Hence, there is an urgent need to find other substances and/or compounds that can attenuate the progression of CKD.
Therefore, investigations of other substances and/or compounds used in traditional medicine across several countries for various ailments were examined. One of the compounds of interest is the Hibiscus sabdariffa Linn. (HS), which is widely cultivated in the tropics such as Asia, South America, and Africa.Citation5 In Thailand, this plant is also known as “Krachiap daeng”. The red persistent flower calyx is often used in health beverages and as a food colorant. In several countries, HS is one of the alternative medicines used to treat gastrointestinal disorders, diaphoresis, and anuria. Substances such as anthocyanin,Citation6 hibiscin, delphinidin-3-glucoside, and cyanidine-3-glucosideCitation7 are believed to exhibit several healing properties and are found in the HS elixirs. As a matter of fact, a combination of several ingredients combining effective properties may actually be responsible for the elixir’s effectiveness.
As a result of this, several studies are investigating the therapeutic uses of HS. For example, HS has been shown to have antibacterial, hypocholesterolemic,Citation8 antioxidant,Citation9 and vasodilatory activities.Citation10,11 In addition, HS was able to lower high blood pressure in hypertensive ratsCitation11 and patients.Citation5 Several activities such as nitric oxide inhibitor,Citation12 calcium channel blocker,Citation13 and angiotensin-converting enzyme inhibitorCitation14 were known to explain the hypotensive effects of HS. Moreover, HS improves the conditions of streptozotocin-induced diabetic nephropathy in rat via several mechanisms such as antihypertensive action, lipid-lowering effect, and antioxidant properties.Citation15 Hence, the multiple effects of HS extract should be effective against multiorgan dysfunctions in CKD patients. However, there are no data on the effects of HS in CKD. Therefore, this study investigated the effects of HS on CKD progression by using a well-established, five-sixth nephrectomy (5/6 Nx) rat model.
METHODS
Animal Model
Male Sprague-Dawley rats (National Laboratory Animal Center, Salaya, Mahidol University, Nakornpathom, Thailand) weighing 200–220 g were given standard diet and housed under standard conditions. All animal protocols complied with the US guidelines (NIH publication #85-23, revised in 1985) for laboratory animal use and care and were approved by the Animal Ethic Committee, Faculty of Science, Burapha University, Chonburi Thailand.
Rats were divided into three groups: (1) HS-treated 5/6 Nx group (Nx+HS), (2) 5/6 Nx-untreated group (Nx), and (3) the sham control group. In the Nx+HS group, the rats had 5/6 nephrectomy and received HS. In the Nx group, nephrectomy was performed and sterile water was given. In the sham control group, sham operation was performed without nephrectomy and sterile water was given. For all groups, the surgery was performed in two stages with ketamine: xylene (5:1) anesthesia as previously described.Citation16 For the Nx+HS and Nx groups, the posterior and anterior apical segmental branches of the left renal artery were ligated at the first stage of the operation (Week −1). One week after the ligation, right nephrectomy was performed as the second stage of the operation (Week 0). For the sham control group, the left renal artery and the right kidney were identified at the first and second stages of the operation, respectively. The timeline of the experiment is shown in .
Figure 1. Timeline of the study. SBP was measured at Week −2 (baseline), Weeks 1, 3, 5, and 7. Renal arterial branch ligation or the first stage of the operation was performed at Week −1. Right nephrectomy or the second stage of the operation was performed at Week 0. The rats were followed for a total of 7 weeks post-nephrectomy. Urine and blood samples were collected at Weeks 0, 1, 3, 5, and 7. All rats were sacrificed at Week 7. Kidney sections were stained for histological examination.
Blood Pressure Measurement
Systolic blood pressure (SBP) was measured by using a tail cuff plethysmography (IITC Life Scientific Instruments, Woodland Hills, CA, USA) as previously described.Citation16 SBP was measured at Week −2 and Weeks 1, 3, 5, and 7. SBP measurements at Week −1 and 0 were omitted due to the confounding of the perioperative stress. SBP measurements were carried out before the start of the experiment to precondition the rats to the procedure. Once the rats were familiar with the process, SBP measurements were obtained three times at each time point with a 10-min rest interval in between the readings. The mean of the three SBP measurements at each time point were used to represent the data.
Preparation of Hibiscus sabdariffa (HS) Aqueous Extract and Drug Administration
The dried calyxes of HS were kindly provided by the Chaophya Abhaibhubejhr Hospital Foundation, Prachinburi, Thailand. Thirty grams of dried HS calyxes were brewed in 200 mL of sterile water and allowed to stand for 30 min. Then the mixture was filtered and the solution was evaporated until dry (Freezer dryer CHRIST; Aoc-1M). From this procedure, 55% of the dark red powder was obtained. The extract was stored at 4°C until use. One week after 5/6 Nx, 250 mg/kg of the powder extract was diluted in 10 mL of sterile water and administered to the Nx+HS group once daily by gavage, whereas only sterile water was given for the Nx and sham control groups.
Collection of Blood and Urine Specimens
Blood samples were taken from the tail vein for the following measurements: blood urea nitrogen (BUN), serum creatinine (Scr), and serum MDA. BUN was measured by colorimetric assay (QuantiChrom Urea assay kit DIUR-500, Hayward, CA, USA). Serum creatinine was measured by picric acid base colorimetric assay (QuantiChrom Creatinine assay kit DICT-500, Hayward, CA, USA). MDA was measured by thiobarbituric acid-reactive substances as previously described (OxiSelect TBARS assay kit, San Diego, CA, USA).Citation17 Enzyme-linked immunosorbent assay (ELISA: R&D Systems, Minneapolis, MN, USA) was used for serum TNF-α and IL-6 levels.
Twenty-four hour (24 h) urine was collected by putting the rats in metabolic cages. Then 24 h urine protein and urine creatinine were measured by using the turbidimetric assay (COBAS Integra 400, IN, USA) and colorimetric assay (QuantiChrom Creatinine assay kit DICT-500), respectively. The creatinine clearance (CCr) was determined by the following formula: 24 h urine creatinine × 24 h urine volume/Scr. Blood samples were collected 1 day before the unilateral nephrectomy (Week 0) and at Weeks 1, 3, and 5 via the tail vein puncture. At Week 7, blood was drawn from the cardiac puncture when the rats were sacrificed. Likewise, 24 h urine samples were collected at Week 0, 1, 3, 5, and 7 at 24 h before blood collection (). At Week 7, all of the rats were sacrificed, then kidney was removed and fixed in 4% paraformaldehyde buffer until use for histology.
Histology
There is one section of kidney per rat for all groups (left 5/6 Nx kidney remnants and left kidney from sham control group). The isolated kidney was fixed with 4% paraformaldehyde and embedded in paraffin. Kidney sections of 4 μm thickness were stained by periodic acid-Schiff (PAS) and Masson’s trichrome. To determine the severity of the kidney injury, the percentage of the glomerular expansion from the PAS staining and the area of the interstitial fibrosis from Masson’s trichrome stained histology were used to gauge the extent of the glomerular injury and renal interstitial damage.Citation18–20 Injured glomeruli were defined as more than 50% expansion of the glomerular lesion at 400× magnification. The severity of the glomerular injury was determined by the percentage of the injured glomeruli (), whereas the renal interstitial injury was estimated by the number of areas with tubulointerstitial expansion at 200× magnification using 10 randomly selected fields for each rat by three pathologists who were blinded from knowing which group the rats belonged to. The semiquantitative criteria used to score the renal interstitial injury were as follows: 0 areas with tubulointerstitial expansion was equivalent to <5% damage, 1 area with tubulointerstitial expansion was equivalent to 5–10% damage, 2 areas with tubulointerstitial expansion was equivalent to 10–25% damage, 3 areas with tubulointerstitial expansion was equivalent to 25–50% damage, and 4 areas with tubulointerstitial expansion was equivalent to >50% damage.Citation18,20 Masson’s trichrome stained sections were used to estimate the kidney interstitial fibrosis at 200× magnification by using the ImageJ 1.36b program (National Institutes of Health, Bethesda, MD, USA) as detailed in http://imagej.nih.gov/ij. In brief, 20 different but randomly selected interstitial areas at 200× magnification without glomeruli and large vessels were captured and converted into black and white colors (HSB stack). Then the percentages of the black particles were analyzed by the ImageJ 1.36b program using the set-up scale from the Richardson test slide (diameter 270 micrometer).
Statistical Analysis
The results are presented as mean ± SD. Statistical differences between the groups were assessed by post hoc ANOVA and Bonferroni’test where appropriate. A p-value <0.05 was considered statistically significant. The statistical calculations were performed by the statistical package SPSS for Windows.
RESULTS
Effects of Hibiscus sabdariffa (HS) Aqueous Extracts on Kidney Injury
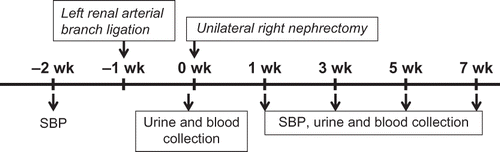
The effects of the reduced kidney mass were found as early as 1 weeks after 5/6 Nx as determined by BUN, Scr, and CCr in both Nx-HS and Nx groups when compared to the sham control group (A–C). At 7 weeks post-5/6 Nx, higher BUN and Scr with reciprocally lower CCr were demonstrated in both Nx-HS and Nx groups than the sham control group (A–C). At 5–7 weeks after 5/6 Nx, the levels of proteinuria started to increase in Nx-HS and Nx groups compared to the sham control group (D). However, 24-h urine protein from the Nx+HS group was not significantly lower compared with the Nx group (D). The results from the PAS staining also showed fewer glomerular and tubulointerstitial injuries in the Nx+HS group at 7 weeks (). According to the Masson’s trichrome staining, there was less kidney fibrosis in the Nx+HS group compared with the Nx group ().
Figure 2. Hibiscus sabdariffa Linn. (HS) attenuated the progression of chronic kidney disease. The progression of the renal injury is shown according to the following measurements: (A) blood urea nitrogen (BUN), (B) serum creatinine (Scr), (C) creatinine clearance (CCr), and (D) 24 h urine protein in HS-treated 5/6 Nx rats (Nx+HS: n = 8), placebo-treated 5/6 Nx rats (Nx: n = 8), and sham control rats (sham: n = 4). Notes: * Indicates that the p < 0.01 is between the Nx-HS or Nx group and the sham group. ** Indicates that the p < 0.05 is between the Nx-HS or Nx group and the sham group. # Indicates that the p < 0.05 is between the Nx-HS and the Nx group.
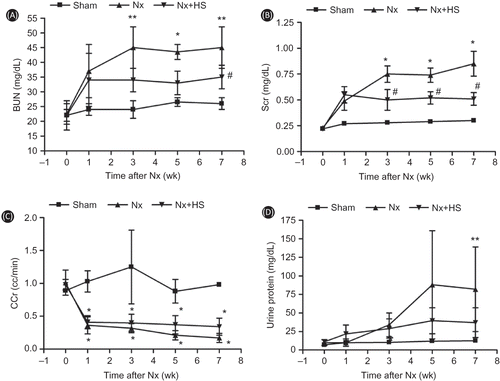
Figure 3. The severity of the chronic kidney disease from renal histology was attenuated by Hibiscus sabdariffa Linn. (HS). The renal histology results are representative of four to eight rats per group. Results from the Periodic Acid-Shiff (PAS) staining of the glomerulus (left panel) and tubulointerstitium (right panel) for the sham control group (sham control rats: A, B), Nx group (placebo-treated 5/6 Nx rats: C, D), and Nx+HS group (HS-treated 5/6 Nx rats: E, F). Magnification of the glomeruli was done at 400X and the interstitial area at 200X. The analysis of the glomerular injury (G) and the tubulointerstitial injury (H) are shown for sham control rats (sham: n = 4), placebo-treated 5/6 Nx rats (Nx: n = 8), and HS-treated 5/6 Nx rats (Nx+HS: n = 8). Note: * indicates p < 0.05.
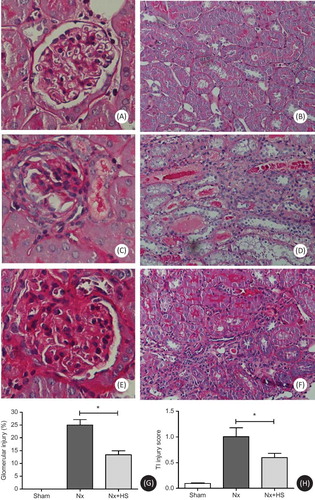
Figure 4. Tubulointerstitial fibrosis in chronic kidney disease is attenuated by Hibiscus sabdariffa Linn. (HS). The renal histology results are representative of four to eight rats per group. Results from the Masson’s trichrome staining for the sham control group (sham control rats: A), Nx group (placebo-treated 5/6 Nx rats: B), and Nx+HS group (HS-treated 5/6 Nx rats: C) at Week 7. Magnification was done at 200X. The quantitative measurement of the tubulointerstitial fibrotic area by image J program (D) are shown for the sham control group (sham: n = 4), placebo-treated 5/6 Nx (Nx: n = 8), and HS-treated 5/6 Nx rats (Nx+HS: n = 8) at 7 weeks after 5/6 Nx.Note: * indicates p < 0.05.
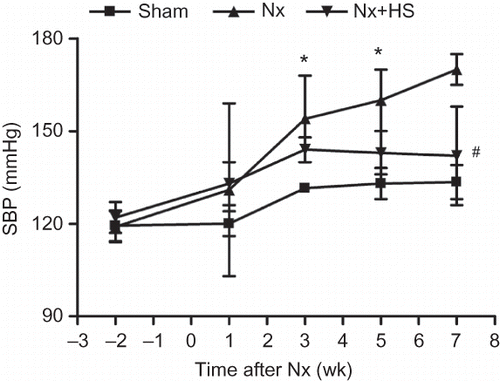
Effects of Hibiscus sabdariffa (HS) Aqueous Extracts on Hypertension
SBP was investigated because hypertension can exacerbate the progression of CKD.Citation18 Three weeks after 5/6 Nx, the SBP from the Nx group was higher than the sham control group (). At Week 7, the SBP from the Nx+HS group was significantly lower when compared with the Nx group (). These results corroborate findings from a previous clinical study.Citation5
Figure 5. Hypertension in chronic kidney disease is attenuated by Hibiscus sabdariffa Linn. (HS). Systolic blood pressure measurements are plotted at multiple time points after 5/6 Nx for the sham control rats (sham: n = 4), placebo-treated 5/6 Nx rats (Nx: n = 8), and HS-treated 5/6 Nx rats (Nx+HS: n = 8).Notes: * Indicates that the p < 0.05 is between the Nx+HS group and the sham group. # Indicates that the p < 0.01 is between the Nx+HS group and the Nx group.
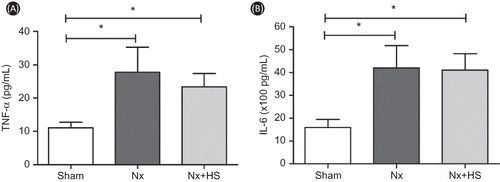
High levels of malondialdehyde (MDA) are usually found in CKD patients. MDA is one of the important uremic toxinsCitation21,22 that is indicative of advanced lipoxidation end-products which is toxic to DNACitation20 and often used as a biomarker for oxidative stress. The levels of MDA in the serum were higher in the Nx group and could be detected as early as 3 weeks. At 5–7 weeks after 5/6 Nx, the MDA levels in the Nx+HS group were significantly lower compared with the Nx group (), but this was not the case for TNF-α and IL-6, which continued to be high even at Week 7 (). Overall, this indicated that the anti-oxidant properties in HS can lower hypertension as early as 7 weeks (), whereas the effects on MDA occurred earlier (5 weeks after 5/6 Nx, ).
Figure 6. The effects of Hibiscus sabdariffa Linn. (HS) in lowering levels of malondialdehyde (MDA) in chronic kidney disease. Serum levels of malondialdehyde (MDA) are plotted at multiple time points for the sham control rats (sham: n = 4), placebo-treated 5/6 Nx rats (Nx: n = 8), and HS-treated 5/6 Nx rats (Nx+HS: n = 8).Notes: * Indicates that the p < 0.05 is between the Nx+HS group and the sham group. # Indicates that the p < 0.01 is between the Nx+HS group and the Nx group. ## Indicate that the p < 0.05 is between the Nx+HS group and the Nx group.
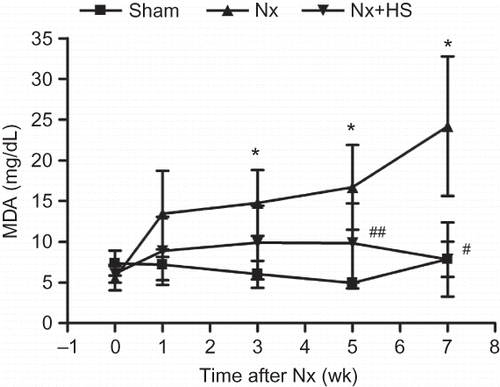
Figure 7. Hibiscus sabdariffa Linn. (HS) had no effect on the serum levels of TNF-α and IL-6 in chronic kidney disease. The serum levels of TNF-α (A) and IL-6 (B) are shown for the sham control rats (sham: n = 4), placebo-treated 5/6 Nx rats (Nx: n = 8), and HS-treated 5/6 Nx rats (Nx+HS: n = 8) at 7 weeks after 5/6 Nx.Note: * indicates p < 0.05.
DISCUSSION
Chronic kidney disease (CKD) is the end stage of renal injury, which can result from several diseases that can impair the normal functions of many organs (e.g., cardiovascular system, hematologic system, etc.) and disturb metabolism (e.g., uremia, anemia, etc.). Currently, there is an increase in the number of patients with CKD worldwide, and this problem is burdening the health care system. The reason for this, at least in part, is because there is no cure for CKD and several combinations of treatments and strategies are required to slow the progression of the disease such as antihypertensive drugs, treatment for anemia, and phosphate control.Citation1 However, the successful attenuation of CKD progression from the current clinical treatment is still limited. Therefore, new medications or compounds are still needed.
Hence compounds from herbal medicine such as Hibiscus sabdariffa (HS) were investigated. This compound is abundantly available in many parts of the world and commonly used as herbal medicine. HS is composed of alkaloids, L-ascorbic acid, anisaldehyde, anthocyanin, β-carotene, β-sitosterol, citric acid, cyanidin-3-rutinoside, delphinidin, galactose, gossypetin, hibiscetin, mucopolysaccharide, pectin, protocatechuic acid, polysaccharide, quercetin, and stearic acid.Citation9 Currently, the dried calyx extracts of HS are commercially available in the form of granules or tea and sold as health food products that are diuretic, hypocholesterolemic,Citation8 antihypertensive,Citation10,11 and an antioxidant.Citation9 Most importantly, HS was recently demonstrated to be effective in attenuating the progression of kidney injury in streptozocin-induced diabetic nephropathy in rats through its antilipidemic and antioxidant properties.Citation15 As a result of this, we investigated the effects of HS using a 5/6 Nx rat model for the study of CKD as a proof of concept for early intervention therapy. HS was administered 1 week after 5/6 Nx.
In our study, we found that HS aqueous extract treatment significantly attenuated the progression of CKD in rats that had 5/6 Nx as measured by BUN, Scr, CCr (A–C), and renal pathology (). However, there was no difference in 24 h proteinuria between the Nx+HS and the Nx groups (D). It is possible that mildly elevated levels of 24 h proteinuria in the rat 5/6 Nx modelCitation23 and/or high variability in the measurement used could have prevented the study from detecting any differences between groups. Longer follow-up period may be needed to observe the differences in proteinuria. Regardless of this, HS was able to attenuate the kidney pathology as determined by PAS and Masson’s trichrome staining ( and ).
Another reason for the HS effectiveness in attenuating CKD is due to its antihypertensive property (). It has been shown that hypertension is the main culprit in exacerbating CKD conditions on top of other factors such as anemia, calciphylaxis, and oxidative stress.Citation1 Therefore, antihypertensive drugs are mandatory for hypertensive CKD patients.Citation1 The most recommended drug of choice for controlling blood pressure in CKD patients is the angiotensin-converting enzyme inhibitorCitation1 of which HS has been shown to have similar activity and potency as the inhibitor in controlling blood pressure in hypertensive patients.Citation5 We hypothesize that this antihypertensive property of HS can attenuate the progression of CKD. In the present study, the systolic blood pressure (SBP) of the Nx+HS group gradually improved after HS administration and reached statistical significance at Week 7 (). It is unclear which mechanisms or pathways is mediated by HS to reduce blood pressure, but it is possible that several mechanisms are involved such as nitric oxide production, calcium channel blocking, and angiotensin-converting enzyme inhibition.Citation12,14
On the other hand, uremic hypertension may be caused by an increase in oxygen free radical activity.Citation24 It has been shown that levels of MDA are directly proportional to the accumulated amount of free oxygen radicals in CKD patients.Citation25,26 It is not entirely clear how the free oxygen radicals are accumulated, but it is possible that a combination of increased production and/or reduced activity is at play. The antioxidant capacity to neutralize the increased free oxygen radicals is also impaired in CKD patients.Citation27,28 Interestingly enough, several other components of HS extract are known to have an antioxidant effect such as anthocyanin, bioflavonoid, protocatechuic acid (PCA), and other phenolic compounds.Citation29 Furthermore, anthocyanin is a far more potent antioxidant than ascorbate (vitamin C), whereas bioflavonoid is one of the well-known robust antioxidants.Citation30 As a result of this, we measured serum levels of oxygen free radical product MDA. We found that serum MDA was significantly lower in the Nx+HS group (). The reduced serum levels of MDA in the Nx+HS group occurred as early as 5 weeks after 5/6 Nx (). This antioxidant effect occurred 2 weeks before changes in the SBP were detected (). We postulate that HS may attenuate the progression of CKD through neutralizing the free oxygen radicals which consequently also reduced uremic hypertension. In addition, it is possible that the antioxidant effect was able to control and lower the inflammation activities of the body resulting in less severe CKD. Several studies have indicated that the severity of CKD is positively correlated with an increased level of inflammatory cytokines.Citation31–34 To determine the HS effect on the inflammatory activities, TNF-α and IL-6 (pro-inflammatory cytokines) were measured at Week 7 after 5/6 Nx (). Both cytokines were mildly elevated in the Nx-HS and Nx group when compared to the sham control group. However, there were no statistical differences in the cytokine levels between the Nx+HS and Nx groups. The comparable cytokine levels between the Nx+HS and Nx groups indicated that the anti-inflammation pathway is not the main protective mechanism of HS in attenuating CKD. Additional studies are needed to delineate the underlying mechanisms of CKD.
In conclusion, we have demonstrated the effectiveness of HS in attenuating the progression of CKD. HS is widely used, well-tolerated, and rarely exhibits any side effect and shows promise in reducing CKD deterioration. Since HS is still effective in attenuating the disease after onset of CKD in our Nx model (HS administration started at Week 1), HS may be an interesting compound for patients with early stage CKD. Larger animal studies and/or proper randomized clinical control trials in CKD patients are warranted.
Declaration Of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
This work was supported by the Research Fund from the Faculty of Science, Burapha University, Chonburi, Thailand, and Grants for Development of New Faculty Staff and Ratchadapiseksompotch fund from the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
REFERENCES
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266.
- Patel SS, Kimmel PL, Singh A. New clinical practice guidelines for chronic kidney disease: a framework for K/DOQI. Semin Nephrol. 2002;22:449–458.
- Mitka M. Report notes increase in kidney disease. JAMA. 2008;300:2473–2474.
- Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12.
- Herrera-Arellano A, Flores-Romero S, Chavez-Soto MA, Tortoriello J. Effectiveness and tolerability of a standardized extract from Hibiscus sabdariffa in patients with mild to moderate hypertension: a controlled and randomized clinical trial. Phytomedicine. 2004;11:375–382.
- Wang CJ, Wang JM, Lin WL, Chu CY, Chou FP, Tseng TH. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem Toxicol. 2000;38:411–416.
- Gowali FM. Hibiscin for mucin. Stain Technol. 1982;57:57–58.
- Hirunpanich V, Utaipat A, Morales NP, . Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J Ethnopharmacol. 2006;103:252–260.
- Hirunpanich V, Utaipat A, Morales NP, . Antioxidant effects of aqueous extracts from dried calyx of Hibiscus sabdariffa Linn. (Roselle) in vitro using rat low-density lipoprotein (LDL). Biol Pharm Bull. 2005;28:481–484.
- Mojiminiyi FB, Dikko M, Muhammad BY, . Antihypertensive effect of an aqueous extract of the calyx of Hibiscus sabdariffa. Fitoterapia. 2007;78:292–297.
- Odigie IP, Ettarh RR, Adigun SA. Chronic administration of aqueous extract of Hibiscus sabdariffa attenuates hypertension and reverses cardiac hypertrophy in 2K-1C hypertensive rats. J Ethnopharmacol. 2003;86:181–185.
- Adegunloye BJ, Omoniyi JO, Owolabi OA, Ajagbonna OP, Sofola OA, Coker HA. Mechanisms of the blood pressure lowering effect of the calyx extract of Hibiscus sabdariffa in rats. Afr J Med Med Sci. 1996;25:235–238.
- Sarr M, Ngom S, Kane MO, . In vitro vasorelaxation mechanisms of bioactive compounds extracted from Hibiscus sabdariffa on rat thoracic aorta. Nutr Metab (Lond). 2009;6:45.
- Jonadet M, Bastide J, Bastide P, Boyer B, Carnat AP, Lamaison JL. [In vitro enzyme inhibitory and in vivo cardioprotective activities of hibiscus (Hibiscus sabdariffa L.)]. J Pharm Belg. 1990;45:120–124.
- Lee WC, Wang CJ, Chen YH, . Polyphenol extracts from Hibiscus sabdariffa Linnaeus attenuate nephropathy in experimental type 1 diabetes. J Agric Food Chem. 2009;57: 2206–2210.
- Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol. 1994;4:2023–2031.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358.
- Leelahavanichkul A, Yan Q, Hu X, . Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model. Kidney Int. 2010;78:1136–1153.
- MacKay K, Striker LJ, Pinkert CA, Brinster RL, Striker GE. Glomerulosclerosis and renal cysts in mice transgenic for the early region of SV40. Kidney Int. 1987;32:827–837.
- Farmer EE, Davoine C. Reactive electrophile species. Curr Opin Plant Biol. 2007;10:380–386.
- Inagi R, Miyata T. Oxidative protein damage with carbohydrates and lipids in uremia: ‘carbonyl stress’. Blood Purif. 1999;17:95–98.
- Siems W, Quast S, Carluccio F, . Oxidative stress in chronic renal failure as a cardiovascular risk factor. Clin Nephrol. 2002;58(Suppl 1):S12–S19.
- Kadowaki D, Anraku M, Tasaki Y, . Evaluation for antioxidant and renoprotective activity of olmesartan using nephrectomy rats. Biol Pharm Bull. 2009;32:2041–2045.
- Vaziri ND, Oveisi F, Ding Y. Role of increased oxygen free radical activity in the pathogenesis of uremic hypertension. Kidney Int. 1998;53:1748–1754.
- Paul JL, Man NK, Moatti N, Raichvarg D. [Membrane phospholipid peroxidation in renal insufficiency and chronic hemodialysis]. Nephrologie. 1991;12:4–7.
- Trznadel K, Pawlicki L, Kedziora J, Luciak M, Blaszczyk J, Buczynski A. Superoxide anion generation, erythrocytes superoxide dismutase activity, and lipid peroxidation during hemoperfusion and hemodialysis in chronic uremic patients. Free Radic Biol Med. 1989;6:393–397.
- Durak I, Akyol O, Basesme E, Canbolat O, Kavutcu M. Reduced erythrocyte defense mechanisms against free radical toxicity in patients with chronic renal failure. Nephron. 1994;66:76–80.
- Turi S, Nemeth I, Vargha I, Matkovics B, Dobos E. Erythrocyte defense mechanisms against free oxygen radicals in hemodialyzed uremic children. Pediatr Nephrol. 1991;5:179–183.
- Seca AM, Silva AM, Silvestre AJ, Cavaleiro JA, Domingues FM, Pascoal-Neto C. Phenolic constituents from the core of kenaf (Hibiscus cannabinus). Phytochemistry. 2001;56:759–767.
- Shirwaikar A, Malini S, Kumari SC. Protective effect of Pongamia pinnata flowers against cisplatin and gentamicin induced nephrotoxicity in rats. Indian J Exp Biol. 2003;41:58–62.
- Fleet M, Osman F, Komaragiri R, Fritz A. Protein catabolism in advanced renal disease: role of cytokines. Clin Nephrol. 2008;70:91–100.
- Stenvinkel P, Heimburger O, Paultre F, . Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911.
- Stenvinkel P, Ketteler M, Johnson RJ, . IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 2005;67:1216–1233.
- Zoccali C, Mallamaci F, Tripepi G. Inflammation and atherosclerosis in end-stage renal disease. Blood Purif. 2003; 21:29–36.
