Abstract
Objective: To investigate the expression and clinical significance of both matrix metalloproteinase-2 (MMP-2) and its tissue inhibitor (tissue inhibitor of metalloproteinase-2 (TIMP-2)) in tunica media of radial artery in uremic patients. Methods: The modified radial arteries from 80 uremic patients during internal arteriovenous fistula surgery were selected and used as experimental specimens. The calcification of tunica media was observed by alizarin red staining, and the expression status of MMP-2, TIMP-2, osteoprotegerin (OPG), and osteopontin (OPN) in tunica media of radial arteries of these patients was detected by immunohistochemical method. The semiquantitative analysis and comparison were conducted based on the calcification grading and the expression of each test protein in tunica media of radial artery. Results: Of the 80 cases of radial artery specimens, 37 cases were presented with various degrees of calcification of tunica media, and the calcification rate was 46.25%; the expression of MMP-2, TIMP-2, OPG, and OPN could be detected in the calcificated tunica media of the radial artery and was positively correlated with the degree of vascular calcification (p < 0.05). Conclusion: The incidence of vascular calcification in uremic patients was high. The occurrence of calcification in tunica media of the radial artery was correlated with the expression of MMP-2 and TIMP-2.
INTRODUCTION
The cardiovascular disease is the most common cause of death in patients with uremia. According to the data system for renal disease in the United States, almost 50% of end stage renal disease (ESRD) patients die of cardiovascular disease. The calcification of arterial tunica media that can lead to increased morbidity and mortality of cardiovascular disease is a sign of vascular disease in patients with uremia.Citation1 The exact etiology and pathogenesis leading to the vascular calcification in patient with uremia are not yet clear. The study on the mechanism of occurrence is important for preventing the clinical events. The matrix metalloproteinases (MMPs) is the most important group of enzymes for the degradation of extracellular matrix (ECM), and their tissue inhibitors (tissue inhibitor of metalloproteinases (TIMPs)) can regulate the in vivo activity of MMPs by changing their own level.Citation2 The studies on the effects of regulation of MMPs/TIMPs in the field of cerebrovascular disease are mainly concentrated in the cerebral hemorrhage/ischemia, atherosclerosis, myocardial remodeling, and so on, with few studies on the effect of vascular calcification. Moreover, the relevant aspects of the MMP-2/TIMP-2 and vascular calcification have rarely been reported, by the detection of MMP-2 and TIMP-2 expression in the tunica media of the radial artery and the analysis of its correlation with vascular calcification.
SUBJECTS AND METHODS
Study Subjects
The subjects were 80 patients with uremia, who received forearm internal arteriovenous fistula surgery during January 2009 to August 2011 in our hospital. There were 46 male cases and 34 female cases, aged 25–83 years, and the mean age was 56.39 ± 13.98 years. Sixty-two patients received the fistula surgery for the first time, and another 18 patients received the second internal fistula surgery. The primary disease included 54 cases of chronic glomerulonephritis, 22 cases of diabetic nephropathy, 1 case of hypertensive renal damage, 2 cases of polycystic kidney disease, and 1 case of chronic tubulointerstitial disease.
Methods
Hematoxylin and Eosin Staining
By xylene conventional dewaxing, the tissues were washed using various levels of ethanol to water, and sectioned: sections were made transparent with xylene and dehydrated using gradient ethanol, rinsed with running water; stained with hematoxylin, counterstained with eosin, again rinsed with running water, dehydrated for transparency, and mounted with neutral gum.
Alizarin Red Staining
The paraffin sections were dewaxed and washed with water. Two percent of alizarin red solution was prepared, with the pH adjusted to 4.1–4.3. The slices were incubated with alizarin red for 3 min, washed with distilled water, counterstained by 0.2% aqueous light green solution for 20–30 s, washed rapidly with acetic acid solution, dehydrated by gradient ethanol, made transparent using xylene, and mounted with neutral gum.
Immunohistochemistry
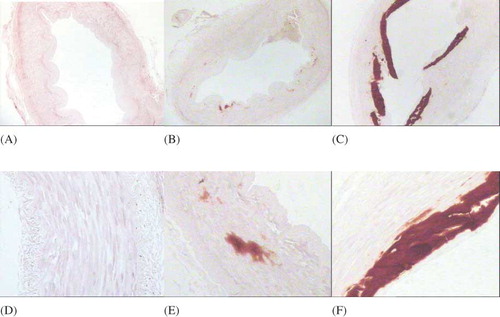
The paraffin sections were dewaxed, hydrated, then blocked for endogenous peroxidase activity with 3% hydrogen peroxide, placed slides in the citrate buffer solution (pH 6.0, 100 mM stock) and microwaved for 10 min at 98°C to retrieve antigen, then blocked nonspecific antigen with normal animal immune serum. Then, the MMP-2, TIMP-2, osteoprotegerin (OPG), and osteopontin (OPN) all antibodies were 1:100 rabbit antihuman antibody, produced by Boster Co., Wuhan, China) were added and incubated at 4°C overnight; then the biotinylated secondary antibody was added and incubated for 30 min at room temperature; the streptavidin–biotin–peroxidase solution was dropped and incubated for 30 min at room temperature; and the 3,3-diaminobenzidine (DAB) coloring reagent was dropped, counterstained by hematoxylin, dehydrated by conventional methods, cleared, and mounted. Phosphate-buffered saline (PBS) was used as negative control instead of primary antibody.
Outcome Indexes
Hematoxylin and eosin staining: To observe the thickness and calcification of tunica media and endangium under the light microscope.
Assessment of pathological findings using alizarin red stainingCitation3: According to the calcium deposition, the pathological findings were divided into 0–4 grading. 0: no calcium deposition; 1: punctate deposition; 2: single flake deposition; 3: multiple flakes deposition; and 4: diffuse deposition around the lumina. Two or three sections were stained for each specimen and the average was taken. The integral under 1 grading was defined as noncalcification; 1–2.5 grading as mild to moderate calcification; over 2.5 grading as severe calcification.
Immunohistochemical staining: Each slice was randomly selected under 10 fields (×400); the staining results were semi-quantitatively analyzed using pathological image analysis software Image-Pro Plus Version 6.0 (Media Cybernetics, Silver Spring, MD, USA) The integrated option density (IOD) value was measured as follows: the IOD = the mean optical density × the total area of image. Ten of results were calculated for the mean and standard deviation.
Statistical Methods
The measurement data were processed using SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA), indicated as (x ± s). Comparisons were done among noncalcification, mild to moderate calcification, and severe calcification groups. The independent samples t-test was used for the comparison of the mean values in two groups of measurement data with normal distribution, and the one-way analysis of variance was used for the comparison of the mean values in multiple groups. The nonparametric test (Spearman’s rank correlation) was used for the relationship between the immunohistochemical expression and vascular calcification. p-Value < 0.05 was considered to be statistically significant.
RESULTS
Hematoxylin and Eosin Staining
Apparent calcification of blood vessels was found in 10 cases under the light microscope, and all the calcification were located in the tunica media. The calcificated endangium was basically complete, with occasionally slight thickening, and the complete internal elastic layer. The thickening of tunica media can be seen and was significant with the increase of calcification. The differences among noncalcification, mild to moderate calcification, and severe calcification groups were statistically significant (p < 0.05). The smooth muscle cells in tunica media without calcification were arranged in linear, whereas the smooth muscle cells in tunica media with calcification showed a haphazard arrangement. Parts of the smooth muscle cells decrease in the number, shown in and .
Figure 1. Hematoxylin and eosin staining of the radial artery.
Notes: The calcification was located in the tunica media. The calcificated endangium was basically complete, and the thickening of tunica media can be seen and was significantly correlated with the increase of calcification. The differences among noncalcification, mild to moderate calcification, and severe calcification groups were statistically significant (p < 0.05).
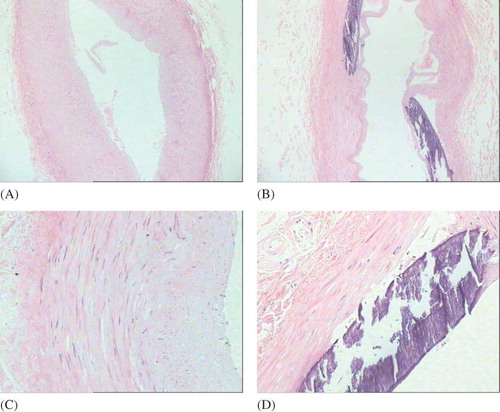
Table 1. Thickness of tunica media in radial artery and IOD values for immunohistochemical staining (n = 80, x ± s).
Alizarin Red Staining
The purple or orange-red calcium deposits can be seen in the calcificated tunica media. Of the 80 cases of radial artery specimens, 37 cases were calcificated, with 46.25% of calcification rate, including 11 cases of severe calcification and 26 cases of mild to moderate calcification, as shown in .
Immunohistochemistry
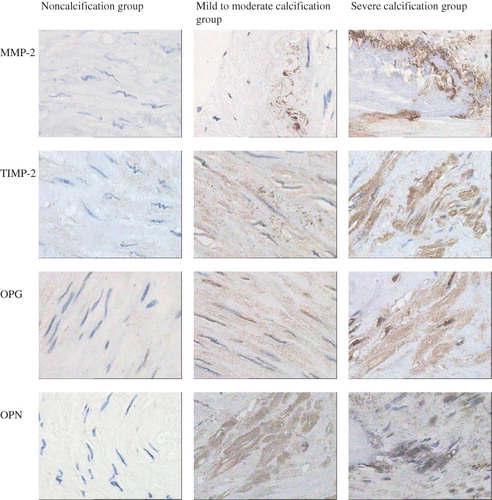
The vascular calcification was significantly correlated with the expression of OPG, OPN, MMP-2, and TIMP-2 (r = 0.204, 0.247, 0.304, and 0.215, respectively, p < 0.05). The expression of OPG, OPN, MMP-2, and TIMP-2 in the cytoplasm of the smooth muscle cells of tunica media gradually increased with the increased calcification, with a statistically significant difference among groups (p < 0.05), as shown in and .
DISCUSSION
The vascular calcification is the most important cause of cardiovascular disease in patients with chronic kidney disease. From the aspects of morphology, there are two classes of the main pathological findings of the calcium and phosphorus deposition in the arterial wall, that is, the intimal calcification and the medial calcification.Citation4 The medial vascular calcification was common in patients with chronic kidney disease, which is an important predictor of cardiovascular disease and all-cause mortality in patients with chronic kidney disease. The study has indicated that the medial vascular calcification can increase vascular stiffness,Citation5 which leads to hypertension, left ventricular hypertrophy, heart failure, and hazards of coronary perfusion.
Traditionally, it has been said that vascular calcification is a passive process caused by an increase of phosphorus and calcium-phosphate product. However, recent studies have found that a number of proteins related to bone metabolism were observed in the arterial tissues, and the calcificated morphology of vascular tissues was similar to that of bone, suggesting that calcium deposition in blood vessels is an active adjustable process similar to bone formation and is also an active process,Citation6,7 which is an exact interaction result during the active and passive processes controlled by local and systemic factors.Citation8
In the process of vascular calcification, ECMs play an important role, and these include elastin, fibronectin, OPN, and osteocalcin. Pai et al.Citation1 considered that elastin remodeling is an early and important process in the pathogenesis of uremic vascular calcification. MMPs are involved in matrix remodeling. MMP-29 is the main protease for artery elastolysis. It can combine and degrade the insoluble elastic protein to produce soluble elastin-derived peptide and also triggers the release of soluble elastin-derived peptides. Soluble elastin-derived peptide can combine with the elastin receptor located on the surface of vascular smooth muscle cells. The interaction between them can induce the transformation of the vascular smooth muscle cells into osteoblast-like cells. During the development of the vascular calcification, the transformation of the vascular smooth muscle cells into osteoblast-like cells is a critical step. Chung et al.Citation9 have found that MMP-2 activity in patients with chronic kidney disease was related to the elastin degradation and vascular calcification. A high degree of correlation between the up-regulated MMP-2 in the vascular system of dialysis patients and disorders of elastic fibers, stiffness, calcification, and vasomotor dysfunction has been reported. Kumata et al.Citation10 have found that elastin degradation and MMP-2 expression can be found in the calcification area of tunica media, suggesting that elastin degradation and MMP-2 expression are involved in vascular calcification. MMP-2 knockout mice were resistant to CaCl2-induced vascular calcification.Citation11
The proteolytic activity of MMPs may be blocked by a variety of endogenous inhibitory factors. TIMPs can inhibit specifically the function of MMPs, with effects of reducing arterial calcification.Citation12 This study has found that the expression of MMP-2 and TIMP-2 in the cytoplasm of medial smooth muscle cells can be increased with the aggravation of calcification, with a statistically significant difference (p < 0.05), suggesting that the MMPs and their inhibitors may be involved in the vascular calcification in uremic patients. Although studies have shown that MMP-2 can promote the occurrence of vascular calcification and TIMP-2 can inhibit the occurrence of vascular calcification, this experiment also found that the expression of both MMPs and TIMPs can be increased with the aggravation of calcification, considering the balance between MMP and TIMP can regulate the speed and quantity of degradation of bone matrix, which can play an important role in maintaining the dynamic equilibrium of ECM, while destroying the dynamic equilibrium between them involved in the vascular tissue remodeling of uremia and the formation of calcification.
OPG is a glycoprotein secreted in ECM that inhibits osteoclast formation. OPN is a major noncollagenous matrix protein of bone, one of secreted phosphorylated glycoprotein combined with calcium, with a variety of functions, can be closely integrated with the hydroxyapatite in tissues, involving in the regulation of calcium deposition in bone. Recently, a number of studiesCitation13–17 have shown that the OPG and OPN levels are positively correlated with vascular calcification, which may be a secondary defense response of the body to calcium and phosphorus deposition and consistent with this study, to inhibit the occurrence and development of vascular calcification, suggesting the role of OPG and OPN in vascular calcification.
In this study, 37 cases of varying degrees of vascular calcification were found, with 46.25% of the calcification rate, suggesting that the incidence of vascular calcification was high in uremic patients. It also can be seen that under the light microscope the tunica media was significantly thickened, with a statistical significance compared with the noncalcification group (p < 0.05). The calcification site was located in the arterious tunica media, and diffused into the elastic, collagen-rich tunica media. The vascular smooth muscle cells can be arranged in disorder, and no significant change was found in tunica intima. The internal elastic layer was complete, and no obvious inflammatory cell infiltration at the lesion area can be found, suggesting that medial calcification began in tunica media, rather than in tunica intima, indicating that the mineralization process was a positive process for transformation of the medial vascular smooth muscle cells into osteoblast-like cells. Compared with atherosclerotic vascular calcification, the location and pathological findings of calcification were different, suggesting that there may be different pathways but basically with the similar molecular mechanisms.Citation18
We have demonstrated that the incidence of vascular calcification is high in patients with uremia and the effects of MMP-2, TIMP-2, OPG, and OPN on the occurrence and development of medial calcification in the radial artery. While we also recognize that our study has some limitations. The population of our study is not a representative of ESRD population, as patients eligible for creating a forearm arteriovenous fistula are usually younger, with less comorbidity and better vessels. Other limitations of the study are that our study is relatively small and lacks a conventional control group. However, although the variability and bias in obtaining the sample cannot be entirely eliminated, they are hopefully reduced as much as possible within the realms of clinical practice.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
REFERENCES
- Pai A, Leaf EM, El-Abbadi M, . Elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification in a uremic mouse model of chronic kidney disease. Am J Pathol. 2011;178:764–773.
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006; 69:562–573.
- Moe SM, O’Neill KD, Duan D, . Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61:638–647.
- Ossareh S. Vascular calcification in chronic kidney disease: mechanisms and clinical implications. Iran J Kidney Dis. 2011;5:285–299.
- Guérin AP, Pannier B, Métivier F, . Assessment and significance of arterial stiffness in patients with chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17:635–641.
- Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059.
- Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453–1464.
- Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536.
- Chung AW, Yang HH, Kim JM, . Upregulation of matrix metalloproteinase-2 in the arterial vasculature contributes to stiffening and vasomotor dysfunction in patients with chronic kidney disease. Circulation. 2009;120:792–801.
- Kumata C, Mizobuchi M, Ogata H, . Involvement of matrix metalloproteinase-2 in the development of medial layer vascular calcification in uremic rats. Ther Apher Dial. 2011;15:18–22.
- Basalyga DM, Simionescu DT, Xiong W, . Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation. 2004;110:3480–3487.
- Qin X, Corriere MA, Matrisian LM, . Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:1510–1516.
- Mesquita M, Demulder A, Wolff F, . Osteoprotegerin and progression of coronary and aortic calcifications in chronic kidney disease. Transplant Proc. 2010;42:3444–3449.
- Kurnatowska I, Grzelak P, Kaczmarska M, . Serum osteoprotegerin is a predictor of progression of atherosclerosis and coronary calcification in hemodialysis patients. Nephron Clin Pract. 2011;117:297–304.
- Shao JS, Sierra OL, Cohen R, . Vascular calcification and aortic fibrosis: a bifunctional role for osteopontin in diabetic arteriosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:1821–1833.
- Mecham RP, Kovacs A, Wang J, . Circulating levels of osteopontin are related with calcification parameters in patients with renal transplantations. Transplant Proc. 2011;43:562–564.
- Rezg R, Barreto FC, Barreto DV, . Inhibitors of vascular calcification as potential therapeutic targets. J Nephrol. 2011;24:416–427.
- Valdivielso JM. Vascular calcification: types and mechanisms. Nefrologia. 2011;31:142–147.