Abstract
Background: Contrast-induced nephropathy (CIN) is one of the most frequent causes of acute renal failure in hospitalized patients with the incremental use of contrast media. We aimed to investigate whether proteinuria may act as a risk factor for CIN in patients with chronic kidney disease. Methods: Seventy hospitalized patients (37 men, 33 women) with chronic kidney disease, proteinuria, and/or estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2, who were exposed to contrast media were investigated prospectively. Thirty patients were diabetic. All patients received prophylaxis against CIN with acetylcysteine and 0.9% intravenous saline. CIN is defined as either a 25% higher increase in serum creatinine (sCr) from the baseline levels or a 0.5 mg/dL increase in sCr at 72 h after contrast media exposure. Results: CIN was detected in 26 (37.1%) patients. Advanced age, diabetes, heart failure, anemia, baseline sCr of >1.5 mg/dL, baseline eGFR of <60 mL/min/1.73 m2, proteinuria of ≥1 g/day, hypoalbuminemia, and the volume of contrast media of ≥100 mL correlated significantly with CIN. The frequency of CIN was significantly higher in patients with proteinuria of ≥1 g/day compared to patients with proteinuria of <1 g/day (p = 0.009). Conclusion: Proteinuria may be a new risk factor for the development of CIN in patients with chronic kidney disease.
INTRODUCTION
Radiological procedures using contrast media are performed by increasing the frequency. This has resulted in an increased incidence of acute renal failure due to contrast-induced nephropathy (CIN).Citation1,2 CIN accounts for 10% of all causes of acute renal failure developed in hospitalized patients, making it the third most common cause.Citation3 Although administration of contrast media usually causes reversible form of acute renal failure, it is not a benign complication.Citation4 CIN can result in prolonged hospital stay, occasional need for dialysis, permanent decrease in residual renal function, and increased mortality.Citation5 Most accepted diagnostic criterion for CIN is a 0.5 mg/dL or 25% rise in serum creatinine (sCr) level within 72 h after exposure to the contrast media. Renal ischemia and direct tubular toxicity of the contrast media are accepted as the main pathophysiological mechanisms of CIN.Citation6,7 In patients without any risk factor, the development of CIN is negligible. Preexisting renal failure, diabetes, advanced age, congestive heart failure, contrast media volume, hypotension, intraaortic balloon pump, and anemia are accepted risk factors for CIN.Citation7
Proteinuria, a well-recognized sign of kidney diseases, is an independent risk factor for the progression of renal failure.Citation8,9 Proteinuria may also accompany chronic kidney disease, diabetes, and heart failure which are accepted risk factors for CIN. In these populations, especially for chronic kidney disease, the impact of proteinuria on CIN is not known. Since proteinuria has toxic effects on tubular system like contrast media, we hypothesized that proteinuria may increase the probability of the development of CIN in patients with chronic kidney disease.
SUBJECTS AND METHODS
Patient Population
Seventy hospitalized patients with chronic kidney disease at predialysis stage, with proteinuria and/or estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2, who underwent an angiographic procedure (coronary angiography or angiography with computed tomography) for evaluation of cardiovascular diseases were prospectively enrolled. Exclusion criteria are as follows: history of myeloproliferative diseases or malign paraproteinemias, history of recent nephrotoxic drug exposure, and ingestion of nonsteroidal anti-inflammatory drugs.
Chronic kidney disease was categorized into five stages based on the classification system established by the National Kidney Foundation Disease Outcomes Quality Initiative (NKF KDOQI): stage 1, signs of kidney damage as evidenced by microalbuminuria or macroalbuminuria and normal GFR (≥90 mL/min/1.73 m2); stage 2, microalbuminuria or macroalbuminuria with mild decrease in GFR (60–89 mL/min/1.73 m2); stage 3, moderate decrease in GFR (30–59 mL/min/1.73 m2); stage 4, severe decrease in GFR (15–29 mL/min/1.73 m2); and stage 5, GFR <15 mL/min/1.73 m2 or dialysis.
We recorded the demographic information of each patient including age, gender, comorbid diseases, and current medication they used. Blood samples for sCr level prior to and 72 h after contrast media exposure were taken. All patients received prophylaxis against CIN with acetylcysteine 1200 mg perorally twice a day starting 24 h before and continuing for 24 h after the procedure and 0.9% intravenous saline with an infusion rate of 1 mL/kg/h starting 12 h before and continuing for 12 h after the procedure. CIN is defined as either a 25% higher increase in sCr from the baseline levels or a 0.5 mg/dL increase in sCr at 72 h after contrast media exposure. In order to analyze the relationship between proteinuria and CIN, patients were divided into two groups according to urinary protein excretion levels: proteinuria of <1 g/day and proteinuria of ≥1 g/day. The study protocol was approved by the local ethics committee. Informed consent was taken from all participants.
Laboratory Measurements
GFR was estimated by the 4-variable Modification of Diet in Renal Disease study equation for each participant. A 24-h urine was collected for the measurement of protein excretion rate in all subjects.
Statistics
Distribution of the variables was checked using the Kolmogorov–Smirnov test. Continuous variables with normal distribution were presented as mean ± SD. Variables with unequal distribution were expressed as medians (interquartile ranges: Q1–Q3). Categorical variables were presented as percentages and numbers. Student’s t-test (if normally distributed) or Mann–Whitney U test (if not normally distributed) were used to compare the continuous variables between the two groups. Categorical variables were compared with χ2-test. Pearson correlation analysis was used to evaluate the relationship between variables. Binary logistic regression analysis was used for multivariate analysis. The odd ratios (ORs) and 95% confidence intervals (CIs) were calculated. Statistical significance was considered at a two-tailed value of p < 0.05. Statistical analyses were performed using SPSS software (Statistical Package for the Social Sciences, version 13.0, SPSS Inc., Chicago, IL, USA).
RESULTS
The mean age of the patients (37 men and 33 women) enrolled in the study was 59.2 ± 9.4 years. Baseline clinical and laboratory characteristics of the patients were listed in . A total of 26 patients (37.1%) developed CIN. Univariate variables associated with CIN are shown in .
Table 1. Baseline demographic and clinical characteristics of the patients.
Table 2. Univariate analysis of the variables associated with CIN.
Table 3. Relationship between urinary protein excretion and CIN in patients with eGFR <60 mL/min/1.73m2.
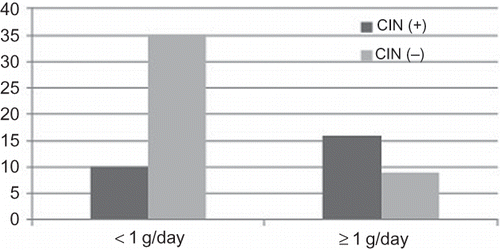
The frequency of CIN was significantly higher in patients with proteinuria of ≥1 g/day compared to patients with proteinuria of <1 g/day (). Frequency of diabetes mellitus and drugs used for hypertension or proteinuria (renin–angiotensin–aldosterone system blockers, beta-blockers, alpha-blockers, diuretics) was similar in both groups. Likewise, CIN was more common in patients with eGFR of <60 mL/min/1.73 m2 and serum albumin levels of <3.5 g/dL. We also evaluated whether proteinuria had an impact on the development of CIN in patient groups with eGFR of ≥60 or <60 mL/min/1.73 m2. In patients with eGFR of ≥60 mL/min/1.73 m2, the frequency of CIN did not differ between patients with proteinuria of ≥1 g/day and <1 g/day. However, for patients with eGFR of <60 mL/min/1.73 m2, CIN was more common in patients with proteinuria of ≥1 g/day compared to patients with proteinuria of <1 g/day ().
In multivariate analysis, the volume of the contrast media (OR = 12.56, 95% CI = 2.920 – 54.019, p = 0.001), eGFR (OR = 0.911, 95% CI = 0.856 – 0.970, p = 0.004), sCr (OR = 0.169, 95% CI = 0.310 – 0.926, p = 0.041), and proteinuria (OR 1.001, 95% CI 1.000 – 1.002, p = 0.011) remained significant independent correlates of CIN.
Marked increment in the sCr (defined as a ≥ 50% and/or ≥1 mg/dL increase in sCr) was detected only in 12 (17%) patients. Among 26 patients who developed CIN, only two patients required hemodialysis and their sCr levels returned to baseline at the time of hospital discharge.
DISCUSSION
In this study, we demonstrated that advanced age, diabetes, heart failure, anemia, baseline sCr of ≥1.5 mg/dL, baseline eGFR of <60 mL/min/1.73 m2, proteinuria of ≥1 g/day, hypoalbuminemia, and contrast media of ≥100 mL correlated significantly with CIN in patients with chronic kidney disease. These variables, except for proteinuria, have been reported as risk factors for the development of CIN in several studies.Citation2,10–12 It was not surprising to find a high frequency of CIN in our study, since the patients enrolled in the study had chronic kidney disease with various risk factors. The incidence of CIN was reported between 4% and 50% in patients with impaired renal function who had additional risk factors.Citation11,12 Although we found a high frequency of CIN, marked increment in the sCr was detected only in 12 (17%) patients.
In this study, we found that proteinuria closely correlated with the development of CIN. In patients with proteinuria of >1 g/day, the frequency of CIN was significantly higher. This relationship was also true in patients who had eGFR of <60 mL/min/1.73 m2. In multivariate analysis, proteinuria along with the volume of the contrast media, sCr, and eGFR remained significant independent factors of CIN. In a study which evaluated CIN in patients with diabetes who underwent coronary angiography, CIN was detected in three of seven patients who had microalbuminuria and all of two patients with overt proteinuria. In patients without proteinuria, no CIN was detected. In the same study, the mean levels of the sCr and proteinuria were significantly higher in patients who developed CIN.Citation13 Recently, Clark et al.Citation14 found that proteinuria (by dipstick evaluation) was the strongest risk factor for the development of CIN in 402 patients with trauma.
The association between proteinuria and CIN can be related to their toxic effects on tubular system. There are several data assuming the direct tubular toxicity of the contrast media as a pathophysiological mechanism for the development of CIN.Citation6,15 Contrast media can also damage renal tubular cells via oxygen radicals.Citation16 Several clinical and experimental studies documented a significant correlation between proteinuria and progression of renal failure.Citation17,18 Proteinuria may cause tubular damage via several mechanisms including the activation of Fas-mediated and peroxisome proliferator-activated receptor-γ-dependent apoptosis and the induction of proinflammatory molecules such as monocyte chemoattractant protein-1, osteopontin, endothelin-1, and interleukin (IL)-8.Citation19–25 Contrast media and proteinuria share similar effects on tubular system injury. It can be hypothesized that contrast media exposure aggregates tubular damage caused by proteinuria.
Some of the limitations in our study are as follows: the number of patients enrolled in our study is small, exposure to contrast media was intraarterial for some patients and intravenous for others, and long-term effects of CIN were not assessed. The findings in our study must be supported by larger studies.
In conclusion, this study demonstrated that proteinuria may be a new undefined risk factor for the CIN in patients with chronic kidney disease, especially in patients with eGFR below 60 mL/min/1.73 m2. Clinicians should be aware of the risk factors to prevent harmful effects of CIN. Special attention should be paid for the development of CIN in patients with proteinuria and reduced GFR.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Guberg L, Mintz GS, Mehran R, . The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36(5):1542–1548.
- McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103(5):368–375.
- Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936.
- Katholi RE, Taylor GJ, McCann WP, . Nephrotoxicity from contrast media: attenuation with theophylline. Radiology. 1995;195(1):17–22.
- Russo D, Minutolo R, Cianciaruso B, Memoli B, Conte G, De Nicola L. Early effects of contrast media on renal hemodynamics and tubular function in chronic renal failure. J Am Soc Nephrol. 1995;6(5):1451–1458.
- Persson PB, Hansell P, Liss P. Pathophysiology of contrast media-induced nephropathy. Kidney Int. 2005;68(1):14–22.
- Mehran R, Aymong ED, Nikolsky E, . A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–1399.
- Burton CJ, Walls J. Proximal tubular cell, proteinuria and tubulo-interstitial scarring. Nephron. 1994;68(3):287–293.
- D’Amico G. Clinical factors in progressive renal injury. The role of proteinuria. Am J Kidney Dis. 1991;17(5 Suppl. 1):48–52.
- Marenzi G, Lauri G, Assanelli E, . Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785.
- Rudnick MR, Goldfarb S, Wexler L, . Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. Kidney Int. 1995;47:254–261.
- Schwab SJ, Hlatky MA, Pieper KS, . Contrast nephrotoxicity: a randomized controlled trial of a nonionic and an ionic radiographic contrast agent. N Engl J Med. 1989;320:149–153.
- Ogi M, Iwase N, Kitamura T, . Risk factors for contrast nephropathy in diabetic patients undergoing cardioangiography. Nippon Jinzo Gakkai Shi. 1993;35:161–170.
- Clark JJ, Wong LL, Lurie F, Kamitaki BK. Proteinuria as a predictor of renal dysfunction in trauma patients receiving intravenous contrast. Am Surg. 2011;77(9):1194–1200.
- Madyoon H, Croushore L, Weaver D, Mathur V. Use of fenoldopam to prevent radiocontrast nephropathy in high-risk patients. Catheter Cardiovasc Interv. 2001;53(3):341–345.
- Goodman AI, Olszanecki R, Yang LM, . Heme oxygenase-1 protects against radiocontrast-induced acute kidney injury by regulating anti-apoptotic proteins. Kidney Int. 2007;72:945–953.
- Remuzzi G. Abnormal protein traffic through the glomerular barrier induces proximal tubular cell dysfunction and causes renal injury. Curr Opin Nephrol Hypertens. 1995;4:339–342.
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17(11):2974–2984.
- Wang Y, Chen J, Chen L, Tay YC, Rangan GK, Harris DC. Induction of monocyte chemoattractant protein-1 in proximal tubule cells by urinary protein. J Am Soc Nephrol. 1997;8(10):1537–1545.
- Erkan E, De Leon M, Devarajan P. Albumin overload induces apoptosis in LLC-PK(1) cells. Am J Physiol Renal Physiol. 2001;280:1107–1114.
- Arici M, Chana R, Lewington A, Brown J, Brunskill NJ. Stimulation of proximal tubular cell apoptosis by albumin bound fatty acids mediated by peroxisome proliferator activated receptor-gamma. J Am Soc Nephrol. 2003;14:17–27.
- Tang S, Leung JCK, Abe K, . Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. J Clin Invest. 2003;111:515–527.
- Zoja C, Morigi M, Figliuzzi M, . Proximal tubular cell synthesis and secretion of endothelin-1 on challenge with albumin and other proteins. Am J Kidney Dis. 1995;26:934–941.
- Cameron JS. Proteinuria and progression in human glomerular diseases. Am J Nephrol. 1990;10(Suppl. 1):81–87.
- Abbate M, Benigni A, Bertani T, Remuzzi G. Nephrotoxicity of increased glomerular protein traffic. Nephrol Dial Transplant. 1999;14:304–312.