Abstract
Introduction: Recurrent urinary tract infection (UTI) is one of the major health problems in children because of its high rate of occurrence. Objective: Our aim of the study was to evaluate the prevalence and determine risk factors of recurrent UTI in Thai children. Patients and Methods: The medical records of children aged less than 15 years diagnosed with UTI at the Department of Pediatrics, Songklanagarind Hospital were reviewed. Results: A total of 307 children (144 boys, 163 girls) were followed up for at least 1 year. Fifty-six children, 31 (19.0%) boys and 25 (17.4%) girls, developed at least one recurrence totaling 153 recurrent UTI episodes. The recurrence rate was not statistically different between the sexes (p = 0.8). On multivariate analysis, genitourinary system (GU) anomalies, particularly vesicoureteral reflux (VUR), were the most significant risk factors. Children aged greater than 5 years had a slightly higher risk of recurrence, irrespective of gender. Comparison of organisms associated with recurrent UTI with those associated with first UTI showed that the prevalence of Escherichia coli decreased from 76.9% to 56.2% but was still the major causative agent. In contrast, the prevalence of Klebsiella pneumoniae and unusual or mixed organisms significantly increased from 7.8% to 15.0% and 6.2% to 16.3%, respectively. Conclusion: One-fifth of children who had UTI developed recurrence and the rates were similar for males and females. Independent risk factors for recurrent UTI were found to be at age of >5 years and underlying disease of either GU anomaly or VUR.
INTRODUCTION
Urinary tract infection (UTI) is one of the most common nephrological problems in general pediatric practice. The possibility of recurrence depends on various factors associated with the individual patient. It has been reported that girls have a higher frequency of recurrent UTI than boys and can be at risk for many years, while recurrent UTI in boys is likely to occur for only a few years after the initial UTI.Citation1,2
The association of UTI in children with genitourinary system (GU) anomalies is well known, particularly primary vesicoureteral reflux (VUR).Citation3,4 Beyond VUR and GU anomalies, some other risk factors have been found associated with recurrent UTI.Citation5,6 The standard treatment for UTI infection in primary VUR patients is antibiotic prophylaxis. Since VUR is the most common abnormality in children with UTI, to prevent recurrences, antibiotic prophylaxis is started immediately after UTI treatment is finished and continued until a voiding cystourethrogram (VCUG) excludes VUR.Citation7 The prophylactic antibiotics are discontinued when VUR has disappeared. The major potential serious consequence of UTI with or without VUR is renal scarring which can cause hypertension and renal failure in later years.Citation3,8,9 Recurrent UTI or primary VUR causes morbidity or urosepsis and has been found to play a major role in renal scarring.Citation10,11 The prevention of recurrent UTI with the associated complications is the golden goal of treating initial UTI; however, achieving this is a big problem because of the many variations possible with age, gender, underlying disease, antibiotic compliance, and other associated factors. The objective of this study was to evaluate the prevalence and determine risk factors of recurrent UTI in Thai children.
PATIENTS AND METHODS
The medical records of patients diagnosed with UTI over a recent 10-year period and followed up for at least 1 year were reviewed. All patients were admitted to the Department of Pediatrics of Songklanagarind Hospital, in southern Thailand. UTI was confirmed by fever and urine culture with significant growth, defined as more than 100,000 colonies/mL from mid-stream or clean-catheterized urine. Children who had not had recurrent UTI and had been followed up for less than 1 year were excluded. Permission from the Institutional Review Board of the university was obtained prior to beginning the study. Statistical analysis was performed using R software, v2.14.1 (R Foundation for Statistical Computing, Vienna, Austria).Citation12 Chi-squared test and Fisher’s exact test were used for comparison among categorical variables. Logistic regression analysis was used to determine independent risk factors for recurrent UTI. The log-rank test was used to compare the survival curves between various groups.
RESULTS
During the 10-year study period, 441 children were diagnosed with first UTI. One hundred and thirty-four children who either did not have a recurrent UTI or were not followed up for at least 1 year were excluded. Three hundred and seven children met the study eligibility criteria. Two hundred and fifty-one children had only one UTI documented in our hospital while 56 children had 153 episodes of recurrent UTI. One girl who first was admitted at the age of 19 weeks with a neurogenic bladder had a total of 40 recurrent UTI episodes throughout a five and a half year period. The ranges of follow-up periods of children with and without recurrent UTI were 2.4 weeks—11.1 years and 1.0–11.3 years, respectively (median 3.3 and 5.3 years, respectively). shows the Kaplan–Meier survival curves indicating the probability of UTI-free survival at different time points (A), stratified by age (B), gender (C), and underlying disease (D). Time to first recurrent UTI ranged from 1 week to 7.4 years (median 14.5 weeks). Time to first recurrence of UTI was significantly different in different age and underlying disease groups, while gender was not a significant factor. Children aged older than 5 years developed recurrent UTI earlier than children aged less than 5 years (p < 0.001). For example, at 5 years, the recurrence rate for children aged ≤5 years was 15.3% while for children aged >5 years the rate was 39.8%.
Figure 1. Kaplan–Meier survival curves showing the probability of recurrence-free survival from UTI at different time periods. The numbers at risk at 2, 5, and 10 years were 229, 135, and 18, respectively. (A) Total cases with 95% confidence interval. (B) Comparison of ages greater and less than 5 years. (C) Comparison of boys and girls. (D) Comparison of underlying diseases.
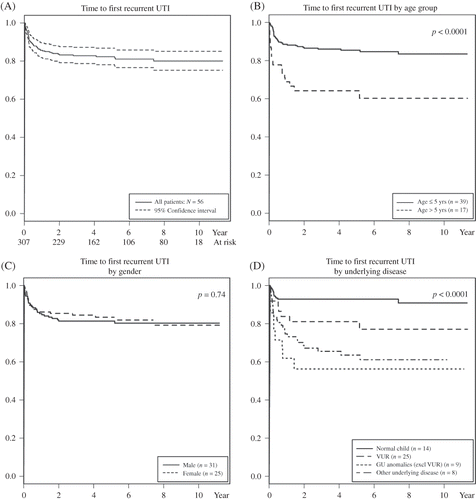
shows the comparisons of age at initial UTI, gender, underlying disease, and causative agent of the initial UTI with and without a recurrence. The ages at diagnosis of UTI for children who had only one UTI episode and those who had recurrent UTI were 3 days—14.2 years versus 1 day—14.5 years, median 1.2 versus 0.8 years, respectively. The children were divided into age groups of 0–6 months, >6–12 months, >1–2 years, >2–5 years, and >5 years. Our results showed that children aged greater than 5 years at initial UTI had a higher percent of recurrent UTI (17/45, 37.8%) compared to the other age groups. Of the 163 boys, 31 (19.0%) developed recurrent UTI and so did 25 of 144 girls (17.4%), (p = 0.8), although overall the number of recurrent UTI episodes was significantly higher in girls than boys (95 vs. 58, p = 0.003). If we exclude the girl who had 40 recurrences, then the number of recurrent UTI episodes in girls and boys was not significantly different (55 vs. 58, p = 0.8). The percent of recurrent UTI was highest among children with GU anomalies (42.9%) followed by VUR (37.3%). Only 7.7% of children without any underlying disease developed recurrent UTI.
Table 1. Characteristics of the 307 patients.
A comparison of all recurrent UTI episodes with the first UTI episode showed that the prevalence of Escherichia coli decreased from 76.9% to 56.2%, but this was still the major causative agent, while the prevalence rates for Klebsiella pneumoniae and unusual organisms significantly increased from 7.8% to 15.0% and 6.2% to 16.3%, respectively ().
Table 2. Characteristics of 460 UTI episodes.
shows the results of the logistic regression analysis, which indicates that there was some confounding by age and gender on underlying disease and causative agents. We found no interaction between age and gender. We combined the age groups for children aged less than 5 into one category because there was no difference within these age groups in terms of recurrent UTI prevalence. Children aged older than 5 years had a higher risk of recurrent UTI than younger children, even after adjusting for other factors. Children with VUR or another GU anomaly had a significant risk of recurrent UTI compared to children with no underlying disease. Children with an underlying disease not related to a GU anomaly did not have a significantly different risk from normal children. Causative agent at initial UTI had no effect on the risk of recurrence. However, the risk of recurrent UTI for children with an unusual organism was significantly higher compared to children with Escherichia coli even after adjusting for underlying disease.
Table 3. Analysis of various factors at first UTI associated with recurrent UTI.
DISCUSSION
Following patients with UTI is important because of the high likelihood of recurrence. In this study, we attempted to determine the prevalence of recurrence(s) and predictive factors in UTI children admitted to our institute. Our primary aim was to try to determine if there is any preventable condition that might alert the physician to a decreased risk of UTI, to avoid potential renal injury from infection, since further infections considerably increase the risk of major renal damage. GU anomalies, including voiding dysfunction, contribute to urine stasis, which is a major factor of both UTI and recurrent UTI.Citation13 Our study showed that once UTI occurs in a child, the chance of recurrence is almost 20% in both boys and girls. GU anomalies, especially VUR, were the most significant independent risk factors. Children aged greater than 5 years at the time of their first UTI are also at greater risk of recurrence.
Although different results concerning recurrence have been found in different studies, such differences can largely be explained by different methodologies, ages of children, and durations of follow-up. Panaretto et al.Citation14 reported a recurrence rate of UTI in normal preschool children of 10%, increasing to 30% in children with VUR. Mingin et al.Citation15 reported that 32.1% (25 out of 78) children with febrile UTI developed recurrent UTI. And recently a report from Italy found only 4.4% of UTI children aged less than 3 years had recurrent UTI after their first UTI.Citation16 In our study, which included all UTI children aged less than 15 years over a 10-year period in our hospital, we found that recurrent UTI in normal children was 7.7%; while in children with a GU anomaly or other underlying disease indicating an immunocompromised host, the recurrence rates were significantly higher. GU anomaly is the major significant risk factor of recurrent UTI; children with a GU anomaly or VUR had recurrence rates of 43% and 37%, respectively. Nuutinen and UhariCitation17 reported that infants aged less than 1 year with grades I and II VUR did not have a significantly different UTI recurrence rate than normal infants, and infants with grades III-V VUR had recurrent UTI more frequently and earlier than infants with low-grade VUR. In contrast, Smellie et al.Citation18 reported that the rates of recurrent UTI were not different between high- and low-grade VUR.
These factors again emphasize that once UTI occurs, radiological investigation is highly advisable to determine the possibility of a GU anomaly, which leads to recurrence in a large number of cases. If there is any possibility of surgical correction or a preventable condition being uncovered, the avoidance of recurrence will be reduced.
Mingin et al.Citation15 reported that in children with febrile UTI, negative renal ultrasound (RUS) and VCUG, girls had a higher recurrence rate than boys. In another study, the age of initial UTI in boys with and without recurrence was not different, but girls with recurrent UTI were younger than girls without recurrent UTI (0.40 vs. 0.52 year, p = 0.008).Citation17 Our study found that the recurrence rate was not statistically different between boys and girls, a result similar to another study in Australian preschool children.Citation14
Our study also found that children first diagnosed with UTI when older than 5 years had a significantly higher rate of recurrence than younger children, although the lower limit of the 95% CI was close to 1.0. In the Australian study, children aged less than 6 months had the greatest risk,Citation14 while age at initial UTI did not predict recurrence in a study of children with febrile UTI and a negative RUS and VCUG.Citation15
VUR was found in 21.8% (67 out of 307) of our cases, which is consistent with other studies.Citation14,19,20 Our study also emphasized that VUR is a major independent risk factor of recurrent UTI, with an odds ratio of 6.6 times that of normal children.
Our study found an overall recurrence rate of 18.2%. For children who had recurrence, the average number of recurrences was 2.7 per child (153/56), which was higher than the number reported in the Australian study [1.4 per child (55/38), an overall recurrence rate of 13.1%].Citation14 A study of recurrent UTI in the United States of America, in infants younger than 6 months with normal RUS and VCUG, found an overall recurrence rate of 20%.Citation21 Another study found recurrent UTI in Belgian children aged 10–14 years to be 25.7% with no difference between genders.Citation5 Daytime wetting, frequent urination greater than 10 times per day and nocturnal enuresis were important risk factors for recurrent UTI in this group of children.Citation5 Unfortunately, this information was not available for a majority of patients in our study, so a comparison could not be made.
In our study, the recurrence rate in younger boys was not higher than in older ones. A comparison of recurrence rates in circumcised and uncircumcised boys was not possible in our study because all boys were uncircumcised since Thailand is a country where routine circumcision is not performed for cultural reasons. However, the recurrence rate did not decline with age in boys, which may mean that phimosis is not a risk factor of recurrent UTI. Other studies have found that the risk of both UTI and recurrent UTI increase in uncircumcised boys aged less than 1 year.Citation15,22
In a study in Finland, children with UTI pathogens other than Escherichia coli were found to have a higher chance of VUR or anatomical or functional defect.Citation23 Our study also found that although Escherichia coli was the major cause of both initial and recurrent UTI, the prevalence declined in recurrent cases compared with the initial UTI (). We also found that recurrent UTI was related to GU anomalies or VUR. The causative agent in recurrent UTI seems to be more related to an associated condition rather than being a true risk factor of recurrent UTI. The causative agents in complicated UTI are somewhat different from simple UTI; therefore, unusual organisms causing UTI may indicate complicated UTI which increases the risk of recurrence.
The management of UTI in our institute is based on established practices; antibiotic prophylaxis is prescribed to children until the abnormalities are resolved, while children who have had at least two episodes are prescribed antibiotic prophylaxis for at least 2 years.Citation24,25 In children with neurogenic bladder, antibiotic prophylaxis is discontinued if a breakthrough infection occurs, which means the antibiotic is not beneficial, and also to avoid drug resistance. The aims of antibiotic prophylaxis in recurrent UTI in children and adults are different. In adults, the aim is to reduce symptomatic cystitis, but in children, the aim is to prevent acute pyelonephritis which might lead to renal scarring.
Some studies have questioned the efficacy of antibiotic prophylaxis in patients with recurrent UTI,Citation6,13 but to date, none have been conclusive. One meta-analysis review concluded that antibiotic prophylaxis “may not” prevent recurrent symptomatic UTI on children.Citation26 A randomized controlled study may be needed to settle the issue. At our institution, we believe efficient and robust follow-up for children who are at risk is the most important concern for immediate diagnosis and treatment of recurrent UTI in order to preserve the kidney.
CONCLUSION
Our 10-year study of a large group of Thai children with UTI demonstrated that recurrence is a serious problem. The long-term health of UTI children needs to be monitored in both normal children and children who have a GU anomaly, even where antibiotic prophylaxis has been prescribed for indicated cases.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- Bergstrom T. Sex differences in childhood urinary tract infection. Arch Dis Child. 1972;47:227–232.
- Winberg J, Bergstrom T, Jacobsson B. Morbidity, age and sex distribution, recurrences and renal scarring in symptomatic urinary tract infection in childhood. Kidney Int. 1975;4(Suppl.):S101–S106.
- Rushton HG. Urinary tract infections in children: epidemiology, evaluation, and management. Pediatr Clin North Am. 1997;44:1133–1169.
- Hellerstein S. Recurrent urinary tract infections in children. Pediatr Infect Dis. 1982;1:271–281.
- Bakker E, van Gool J, van Sprundel M, van der Auwera JC, Wyndaele JJ. Risk factors for recurrent urinary tract infection in 4332 Belgian schoolchildren aged between 10 and 14 years. Eur J Pediatr. 2004;163:234–238.
- Garin EH, Campos A, Homsy Y. Primary vesicoureteral reflux: review of current concepts. Pediatr Nephrol. 1998;12:249–256.
- ; Indian Society of Pediatric NephrologyVijayakumar M, Kanitkar M, Nammalwar BR, Bagga A. Revised statement on management of urinary tract infections. Indian Pediatr. 2011;48:709–717.
- Jacobson SH, Eklof O, Eriksson CG, Lins LE, Tidgren B, Winberg J. Development of hypertension and uraemia after pyelonephritis in childhood: 27 year follow up. Br Med J. 1989;299:703–706.
- Vachvanichsanong P. Urinary tract infection: one lingering effect of childhood kidney diseases—review of the literature. J Nephrol. 2007;20:21–28.
- Merrick MV, Notghi A, Chalmers N, Wilkinson AG, Uttley WS. Long-term follow up to determine the prognostic value of imaging after urinary tract infections. Part 1: Reflux. Arch Dis Child. 1995;72:388–392.
- Merrick MV, Notghi A, Chalmers N, Wilkinson AG, Uttley WS. Long-term follow up to determine the prognostic value of imaging after urinary tract infections. Part 2: Scarring. Arch Dis Child. 1995;72:393–396.
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: www.R-project.org. Accessed December 1, 2010.
- Beetz R. May we go on with antibacterial prophylaxis for urinary tract infections? Pediatr Nephrol. 2006;21:5–13.
- Panaretto K, Craig J, Knight J, Howman-Giles R, Sureshkumar P, Roy L. Risk factors for recurrent urinary tract infection in preschool children. J Paediatr Child Health. 1999;35:454–459.
- Mingin GC, Hinds A, Nguyen HT, Baskin LS. Children with a febrile urinary tract infection and a negative radiologic workup: factors predictive of recurrence. Urology. 2004;63:562–565.
- Pennesi M, L’erario I, Travan L, Ventura A. Managing children under 36 months of age with febrile urinary tract infection: a new approach. Pediatr Nephrol. 2012;27:611–615.
- Nuutinen M, Uhari M. Recurrence and follow-up after urinary tract infection under the age of 1 year. Pediatr Nephrol. 2001;16:69–72.
- Smellie JM, Prescod NP, Shaw PJ, Risdon RA, Bryant TN. Childhood reflux and urinary infection: a follow-up of 10–41 years in 226 adults. Pediatr Nephrol. 1998;12:727–736.
- Sargent MA. What is the normal prevalence of vesicoureteral reflux? Pediatr Radiol. 2000;30:587–593.
- Drachman R, Valevici M, Vardy PA. Excretory urography and cystourethrography in the evaluation of children with urinary tract infection. Clin Pediatr (Phila). 1984;23:265–267.
- Bratslavsky G, Feustel PJ, Aslan AR, Kogan BA. Recurrence risk in infants with urinary tract infections and a negative radiographic evaluation. J Urol. 2004;172:1610–1613.
- Singh-Grewal D, Macdessi J, Craig J. Circumcision for the prevention of urinary tract infection in boys: a systematic review of randomised trials and observational studies. Arch Dis Child. 2005;90:853–858.
- Lomberg H, Hellstrom M, Jodal U, Leffler H, Lincoln K, Svanborg Edén C. Virulence-associated traits in Escherichia coli causing first and recurrent episodes of urinary tract infection in children with or without vesicoureteral reflux. J Infect Dis. 1984;150:561–569.
- Williams G, Craig JC. Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst Rev. 2011;16(3):CD001534.
- Craig JC, Simpson JM, Williams GJ, . Prevention of recurrent urinary tract infection in children with vesicoureteric reflux and normal renal tracts (PRIVENT) investigators. Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2009;361:1748–1759.
- Pérez-Gaxiola G. Review: antibiotic prophylaxis may not prevent recurrent symptomatic urinary tract infection in children. Arch Dis Child Educ Pract Ed. 2011;96:198. doi:10.1136/adc.2011.214551.
