Abstract
Introduction: Measuring total (residual kidney plus peritoneal) creatinine clearance (CrCl) with 24-h urine and dialysate collections is recommended for peritoneal dialysis (PD) adequacy evaluation. Prediction equations applied in this instance could simplify the approach. Cockcroft-Gault and modification of diet in renal disease (MDRD) four (MDRD-4) and six (MDRD-6) variables equations have been tested in this setting, and conflicting results have been reported. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is currently considered to be more sensitive than the established equations for kidney function estimation. However, its performance in PD adequacy evaluation has not been studied. Our aim was to assess CKD-EPI equation’s performance in predicting total measured CrCl (MCC) in PD patients. Material and methods: A group of 23 consecutive PD patients, male/female: 5/18, median age: 66 (32–91) years, median time on PD 32 (2–126) months, were enrolled in the study. All were treated by automated PD (APD). Sixteen out of twenty-three had residual renal function (RRF). MCC was determined from 24-h dialysate and urine collections and also predicted by Cockcroft-Gault, MDRD (4 and 6), and CKD-EPI equations. Results: CKD-EPI and MDRD-6 estimation results were similar to MCC (9.01 ± 3.90 and 9.54 ± 2.98 vs. 8.64 ± 3.75 mL/min/1.73 m2 p = 0.49 and 0.09, respectively). Neither the presence nor the volume of residual urine affected the accuracy of prediction. Cockcroft-Gault and MDRD-4 equations differed significantly from MCC and were not accurately predictive. Conclusion: CKD-EPI equation could be used with accuracy for predicting MCC in PD patients. Only MDRD-6 showed similar accuracy, whereas MDRD-4 and Cockcroft-Gault equations were found to be inappropriate in this setting.
INTRODUCTION
Peritoneal dialysis (PD) is a well-known and widely used form of chronic renal replacement therapy (RRT). PD adequacy exerts significant influence on clinical outcomes, and its assessment is an integral component of chronic PD patients’ evaluation and management.Citation1 The Kidney Disease Outcome Quality Initiative Clinical Practice Guidelines for PD Adequacy 2006 recommend that the total solute clearance should be measured within the first month after initiating dialysis therapy and at least once every 4 months thereafter.Citation2 However, this is a rather cumbersome, time-consuming procedure that involves collecting dialysate and urine simultaneously over a 24-h period, sending the samples to the laboratory for analysis together with a blood sample, then interpreting the results and calculating the adequacy indexes. Furthermore, the analysis of the samples incurs extra expenses in patient care. Finally, results depend on patient compliance in the test period and on the accuracy of timed collections.Citation3
A simple, inexpensive tool requiring no fluid collection to enable assessment of PD adequacy once a month or, in general, whenever it is indicated on clinical grounds would be welcome, in an attempt to monitor PD patients more closely, and in particular those at risk of inadequate dialysis. Creatinine-based GFR-estimating equations, widely used for renal function prediction in pre-dialysis chronic kidney disease (CKD) patients, are theoretically capable of predicting measured total (residual kidney plus peritoneal) creatinine clearance (CrCl) in PD patients.Citation3 Unlike hemodialysis (HD), PD is a continuous form of treatment and, thus, PD patients are in a near-steady metabolic state, similar to pre-dialysis CKD. Indeed, this is the rationale for using GFR-estimating equations in assessing PD adequacy. Cockcroft-Gault (C-G)Citation4 and Modification of diet in renal disease (MDRD) study four (MDRD-4) or six (MDRD-6) variablesCitation5 equations, the most widely used for clinical and research purposes, have already been tested in this setting, with variable accuracy.Citation6–10 Inherent limitations of these equations along with special characteristics of this patient population account for the conflicting results.
The recently developed and validated CKD Epidemiology Collaboration (CKD-EPI) equation was derived from a more diverse population compared with that of the MDRD equation and was thus proposed to improve the accuracy of GFR estimation across a wide variety of populations and clinical conditions.Citation11 Subsequently, several studies have concluded that the CKD-EPI equation could replace the widely used MDRD study equation for general clinical use and throughout the GFR range.Citation11,12 However, its performance in the estimation of PD adequacy has not been evaluated as yet.
The aim of this study was to prospectively determine whether CKD-EPI equation could provide an accurate prediction of total measured CrCl (MCC) in PD patients.
MATERIAL AND METHODS
A group of 23 consecutive unselected PD patients under follow-up in a single PD unit were enrolled in the study. All were on APD with an icodextrin daytime dwell. Sixteen out of twenty-three had RRF (daily urine volume >100 mL). Patients’ characteristics are shown in . The study was performed in accordance with the Declaration of Helsinki and with the approval of the local ethics committee. All patients gave informed written consent before enrollment.
Table 1. Characteristics of the study patients.
Table 2. MCC and prediction equations results according to the presence and the volume of RRF.
MCC was determined from 24-h dialysate and urine collections and also predicted by C-G, MDRD-4, MDRD-6, and CKD-EPI equations.
24-hour dialysate collections were performed at home, and a serum creatinine value was obtained the following morning to calculate peritoneal CrCl. Urine, if present, was also simultaneously collected to determine residual renal CrCl that was calculated as the mean of renal creatinine and urea clearances. The sum of peritoneal CrCl and residual renal CrCl was the total MCC, the “gold standard” of small molecule clearance that was used in the analysis. These data were originally reported as weekly CrCl in liters/week and were converted for this study into mL/min/1.73 m2 by dividing the weekly CrCl by 10,080 (the number of minutes in a week) and normalizing for body surface area (BSA), which was calculated for each patient by using the Dubois formula.Citation13 MCC was determined for each patient on two consecutive monthly occasions, and the mean of these two values was used for the analysis. Detailed instructions to ensure correct urine and dialysate collections were given, and patient compliance was systematically checked. Four creatinine-based equations were tested for their accuracy in predicting total MCC in our PD patients: C-G,Citation4 MDRD-4,Citation5 MDRD-6,Citation5 and CKD-EPI.Citation11 The results of the last three equations were expressed in mL/min/1.73 m2, whereas in C-G equation the results were originally presented in mL/min. To allow comparisons, C-G estimates were computed adjusted to 1.73 m2 BSA by using the Dubois formula.Citation13
Creatinine levels (mg/dL) were measured both in dialysate and in urine and serum samples using an isotope dilution mass spectrometry (IDMS)-traceable kinetic Jaffé method (Thermo Scientific Konelab Prime 60i Clinical Chemistry Analyzer). Blood urea nitrogen (BUN) and serum albumin required for MDRD-6 were measured using standard laboratory methods.
All data are presented as mean ± SD. Pairwise comparison of the mean difference between MCC and prediction equations was performed using paired t-test. Correlation was assessed by Pearson coefficient. Bias was defined as the mean difference between estimated and measured kidney function, whereas precision was expressed as the standard deviation (SD) of this difference. Accuracy was defined by the percentage of GFR estimates lying within 30% of the MCCCitation14 and is the best overall measure for comparing different GFR-estimating equations integrating both bias and precision. Differences in bias and accuracy were tested with paired t-test and McNemar test, respectively. Furthermore, Bland-Altman analysis was performed to assess the mean difference (bias) and the limits of agreement (bias ± 1.96 SD) between MCC and GFR-estimating equations.Citation15 A positive difference suggests an overestimation by the equation, whereas a negative difference an underestimation. p-Values < 0.05 were considered to be statistically significant.
RESULTS
The two monthly measurements of total MCC reported in all our study patients differed from each other less than 15%. The mean MCC for the study group was 8.64 ± 3.75 mL/min/1.73 m2.
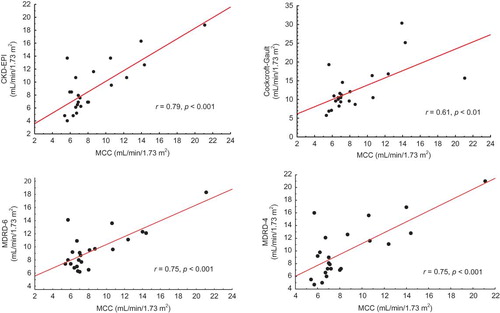
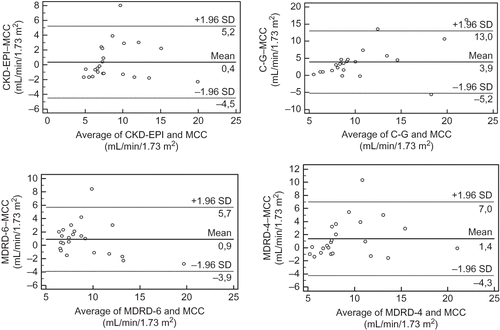
CKD-EPI and MDRD-6 equations predicted MCC reasonably well (9.01 ± 3.90 and 9.54 ± 2.98 vs. 8.64 ± 3.75 mL/min/1.73 m2, p = 0.49 and p = 0.09, respectively), with an accuracy of 95.6% for both equations. In contrast, the mean values predicted by C-G and MDRD-4 equations were 12.53 ± 5.88 and 10.00 ± 4.27 mL/min/1.73 m2, respectively, and differed significantly from MCC (p < 0.001 and p = 0.03, respectively). These equations had an accuracy of 82.6% and 87%, respectively, that did not differ significantly (p = 0.06, McNemar test). On the contrary, accuracy was significantly higher for both CKD-EPI and MDRD-6 than for C-G (p = 0.0014) and MDRD-4 (p = 0.03). There was a strong correlation between MCC and all prediction equations studied (CKD-EPI: r = 0.79, MDRD-6, and MDRD-4: r = 0.75, p < 0.001 in all cases), with the exception of C-G where the correlation was moderate (r = 0.61, p < 0.01). The scatterplots are shown in . The differences between MCC and GFR-estimating equations, as assessed by Bland-Altman analysis, were plotted against the average of the two methods and are shown in . The mean difference (bias) was significantly smaller for CKD-EPI (0.4, limits of agreement −4.5 and 5.2 mL/min/1.73 m2) and MDRD-6 (0.9, −3.9, and 5.7 mL/min/1.73 m2) as compared to C-G (3.9, −5.2, and 13.0 mL/min/1.73 m2, p < 0.001 for both comparisons) and MDRD-4 (1.4, −4.3, and 7.0 mL/min/1.73 m2, p < 0.001 for both comparisons).
Figure 2. Bland–Altman plots of GFR-estimating equations and MCC. The solid lines represent the mean difference and the dashed lines the limits of agreement between the two measurements.
For patients with RRF, the mean 24-h urine volume was 680.00 ± 250.67 mL. Values for the actual MCC and those predicted by the GFR-estimating equations in patients with larger (n = 6) or smaller (n = 10) than the 680-mL daily urine volumes are shown in . Patients with RRF were also compared to the anuric ones, and the results are shown in the same table. Neither the presence nor the volume of residual urine appeared to affect the accuracy of the prediction.
DISCUSSION
The results of this study suggest that CKD-EPI equation could provide an accurate estimation of CrCl delivered in PD patients. Subsequently, PD adequacy could be evaluated with reasonable precision. Only MDRD-6 showed similar accuracy, whereas MDRD-4 and Cockcroft-Gault were found to be inappropriate in this setting. Although CKD-EPI has been shown to estimate GFR more accurately than MDRD-4,Citation12 its performance in dialysis patients is unknown, and this is the first study with CKD-EPI equation applied to PD patients.
Generally, GFR-estimating equations may represent useful tools in the assessment of dialytic clearance and RRF in dialysis-dependent subjects. However, they have not been adequately validated in this unique patient population, and their application may have several notable limitations.Citation16 The presence of a steady metabolic state with stable creatinine concentration and distribution is a prerequisite for equation result validity. Subsequently, the use of GFR-estimating equations is not only clearly unreliable in patients with acute kidney injury and rapidly changing renal function, but may also be inadequate in patients with variable serum creatinine concentrations such as those requiring intermittent RRT. Not unexpectedly, most studies to date have failed to show a reliable performance of GFR-estimating equations in HD patients.Citation16,17
PD patients, in contrast to their HD counterparts, have a stable serum creatinine concentration and, therefore, creatinine-based GFR-estimating equations are theoretically suitable for predicting total MCC in this patient population. Very few studies have evaluated to date the performance of these equations in predicting PD-delivered CrCl. However, it is generally acknowledged that creatinine removal in the peritoneal fluid may result in GFR overestimation by creatinine-based prediction equations,Citation16 in line with our findings regarding C-G and MDRD-4 equations. Furthermore, changes in nutritional and muscle mass status, particularly prevalent in dialysis patients, may induce lower serum creatinine values and a subsequently fictitious increase in the total MCC estimate, providing additional explanation for the abovementioned findings.
Taskapan et al.Citation18 examined the accuracy of MDRD-4 and C-G equations in predicting total MCC in patients on chronic PD. In 156 measurements, they found that the C-G equations overestimated total MCC, in agreement with our findings. However, their mean MDRD results were not significantly different from mean total MCC in the overall cohort, and in males, whereas in female patients MDRD-4 significantly overestimated MCC.
C-G and MDRD-4 equations have been extensively used for clinical purposes. Their comparative performances have been assessed in numerous studies and in various populations, with the majority of them showing superiority of the latter in GFR prediction.Citation3,8–10,16 Generally C-G equation tends to overestimate the effect of age on creatinine excretion and, according to the literature, it is not suitable for predicting MCC in PD patients.Citation10 Indeed, C-G equation was found to be inaccurate in predicting PD adequacy in a large cohort of Scottish patientsCitation10 and in a multicenter Italian study.Citation3 Our results regarding C-G equation are in agreement with these studies.
Data on MDRD-4 and -6 equations’ performance in PD patients are inconclusive. However, most of the studies to date do not support the applicability of these equations in patients with advanced renal failure, including those on dialysis. A plausible explanation is that in these subjects low muscle mass is a relatively more important determinant of plasma creatinine than real GFR.Citation17 MDRD-4 equation was found to be unreliable in estimating total MCC in a large cohort of Scottish patients, in agreement with our results.Citation10 Likewise, a recent multicenter Dutch study showed that MDRD-4 was not suitable for GFR estimation in CKD stage 5 patients at the start of dialysis.Citation17 Generally, MDRD-4 appeared to overestimate true GFR in these studies, a result consistent with ours. On the contrary, MDRD-4 showed good performance in assessing PD adequacy in a multicenter Italian study.Citation3 Both MDRD-4 and -6 equations were found to estimate MCC in a large cohort of PD patients with reasonable accuracy, comparable to their performance in pre-dialysis patients.Citation7 MDRD equations’ better performance as compared to C-G was attributed by the authors of this study not only to the accurate prediction of creatinine production rates from demographic determinants but also to the exponential transformation of serum creatinine to account for the increasing intestinal metabolism of creatinine as its serum concentration rises. Furthermore, these authors’ findingsCitation7 in relation to RRF and accuracy of the prediction are also in agreement with ours.
MDRD-6 equation, in contrast to MDRD-4, appeared to accurately predict MCC in our patients. The discrepancy between these equations is inconsistent with previous published results, showing that both are either equally accurate in predicting PD-delivered CrClCitation7 or equally inaccurate in estimating GFR in CKD 5 patients at the start of dialysis.Citation17 The inclusion of BUN and albumin in MDRD-6 did not seem to result in an improved performance.Citation17 However, significant changes in body composition due to fluid retention and in nutritional status, particularly common in dialysis patients, may be more accurately detected by MDRD-6 and, subsequently, provide an explanation for better performance of the latter in our study. Of note, MDRD-6 also showed a better than MDRD-4 performance in estimating GFR in patients with cirrhosis, another special population with abnormal BUN and albumin levels.Citation19 However, as most studies to date have evaluated only the abbreviated MDRD-4 equation, further data are needed to clarify MDRD-6 equation’s performance in PD patients.
Our study’s novel approach consists in the application of CKD-EPI to PD patients. This equation was derived from a dataset consisting predominantly of young or middle-aged participants with a robust representation of patients with mild to moderate kidney disease, resulting in a mean measured GFR of 68 mL/min in the development and the validation cohort.Citation11,12 This equation uses the same four variables (creatinine, age, sex, and race) as MDRD-4, but applies a different mathematical model, allowing for better estimation of measured GFR throughout its whole range. Its performance has been evaluated in various groups of patients with largely encouraging results.Citation20 The validation process of CKD-EPI equation showed a substantially improved performance for estimating GFR levels above 60 mL/min/1.73 m2 and a similar performance for eGFR below 60 mL/min/1.73 m2 compared to MDRD.Citation12,21,22
To the best of our knowledge, no studies to date have evaluated the performance of CKD-EPI equation in PD patients. Furthermore, there are limited data in advanced CKD. A recent study in 89 nondialysed CKD 4 or 5 patients reported CKD-EPI accuracy equivalent to MDRD-4.Citation23 This is in contrast to our findings that showed superiority of CKD-EPI to MDRD-4 equation, although applied in a different population. However, this study,Citation23 in line with ours, showed an accurate predictive performance of CKD-EPI in a special subgroup of patients. Furthermore, the limited accuracy of C-G equation resulting in overestimation of GFR was confirmed in the moderate-to-severe CKD patients of this studyCitation23 in agreement with our findings in PD patients.
The performance of C-G, MDRD-4, and CKD-EPI equations in relation to GFR was examined in 271 subjects undergoing a GFR measurement with 125I-iothalamate in a Dutch academic medical center.Citation24 Comparable results were found for both MDRD-4 and CKD-EPI in the overall cohort. In the subgroup of subjects with measured GFR 15–29 mL/min/1.73 m2 (CKD4), MDRD-4 equation showed the highest accuracy, with similar results for CKD-EPI equation, partially in the opposite of our findings, although the comparison is questionable due to the different populations studied.
Recently, in a cohort of patients after renal transplantation with mean GFR measured by 99mTc-DTPA clearance at 39.6 (37.3–42.0) mL/min/1.73 m2 the diagnostic performance of CKD-EPI equation in comparison to MDRD was questioned as CKD-EPI led to a considerable overestimation of GFR and less accurate results than MDRD.Citation25
A limitation of the present study is the small sample size, which might result in some marginal statistical differences and discordant findings that cannot be easily interpreted. Furthermore, although dialysate and urine collections still represent the gold standard for assessing PD adequacy, potential day-to-day variability in measurements, particularly in those patients with significant RRF, could have influenced our results. Nevertheless, the two monthly measurements of total MCC we applied to all our study patients did not differ from each other by more than 15%.
In conclusion, our study suggests that the recently proposed CKD-EPI equation showed a reliable performance in predicting total MCC and, subsequently, in assessing PD adequacy. MDRD-6, in contrast to MDRD-4 and C-G, was similarly found to be accurate in this setting. CKD-EPI was proposed by experts in the fieldCitation11 to replace MDRD, the most widely used GFR-estimating equation, in all clinical settings. However, data on the performance of CKD-EPI in patients with advanced renal failure are limited, and no study as yet has applied this equation to PD patients. Our pilot study’s preliminary results could justify further testing and confirmation in a larger population in order to conclusively determine the potential role of CKD-EPI equation in defining treatment adequacy in PD patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Churchill DN, Taylor DW, Keshaviah PR. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. J Am Soc Nephrol. 1996;7:198–207.
- National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for 2006 updates: hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis. 2006;48(Suppl. 1):S1–S322.
- Virga G, La Milia V, Russo R, Bonfante L, Cara M, Nordio M. Comparison between creatinine-based equations for estimating total creatinine clearance in peritoneal dialysis: a multicentre study. Nephrol Dial Transplant. 2010;25:262–269.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
- Levey AS, Bosch JP, Breyer Lewis J, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470.
- Stevens L, Coresh J, Greene T, Levey A. Assessing kidney function: measured and estimated glomerular filtration rate. N Eng J Med. 2006;354:2473–2483.
- Khosla N, Steiner RW. MDRD equation predicts peritoneal dialysis-delivered creatinine clearances from serum creatinine. Clin J Am Soc Nephrol. 2009;4:798–803.
- Jones CH, Newstead CG, Will EJ. Estimation of total creatinine clearance in CAPD from serum creatinine concentration. Perit Dial Int. 1997;17:250–254.
- Kuan Y, Hossain M, Surman J, El Nahas AM, Haylor J. GFR prediction using the MDRD and Cockcroft and Gault equations in patients with end-stage renal disease. Nephrol Dial Transplant. 2005;20:2394–2401.
- Traynor JP, McManus SK, Mactier RA. Derived equations are not precise enough to predict the adequacy of creatinine clearance in peritoneal dialysis patients. Am J Kidney Dis. 2002;40:1036–1044.
- Levey AS, Stevens LA, Schmid CH, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604–612.
- Stevens LA, Schmid CH, Greene T, . Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56:486–495.
- Du Bois D, du Bois EF. A formula to estimate the approximate surface area if height and weight are known. Arch Intern Med. 1916;17:863–871.
- Levey AS, Coresh J, Balk E, . National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification and stratification. Ann Intern Med. 2003;139:137–147.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310.
- White CA, Akbari A. The estimation, measurement and relevance of the glomerular filtration rate in stage 5 chronic kidney disease. Semin Dial. 2011;24:540–549.
- Grootendorst DC, Michels WM, Richardson JD, . The MDRD formula does not reflect GFR in ESRD patients. Nephrol Dial Transplant. 2011;26:1932–1937.
- Taskapan H, Theodoros P, Tam P, Bargman J, Oreopoulos D. Glomerular filtration rate (GFR) estimated from serum creatinine predicts total (urine and peritoneal) creatinine clearance in patients on peritoneal dialysis. Int Urol Nephrol. 2010;42:1085–1092.
- Chen YW, Chen HH, Wang TE, Chang CW, Chang CW, Wu CJ. Difference between CKD-EPI and MDRD equations in calculating glomerular filtration rate in patients with cirrhosis. World J Gastroenterol. 2011;17:4532–4538.
- Winearls CG, Lamb EJ. The CKD-EPI equation to estimate GFR-better alchemy? Nat Rev Nephrol. 2011;7:127–128.
- Liborio AB, Barros RM, Esmeraldo RM, Oliveira ML, Silva GB Jr, Daher EF. Creatinine-based equations predicting chronic kidney disease after kidney donation. Transplant Proc. 2011; 43:2481–2486.
- Ibrahim H, Mondress M, Tello A, Fan Y, Koopmeiners J, Thomas W. An alternative formula to the Cockcroft-Gault and the Modification of Diet in Renal Disease formulas in predicting GFR in individuals with type 1 diabetes. J Am Soc Nephrol. 2005;16:1051–1060.
- Briones JLT, Couto AG, Sabater J, . Validation of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in advanced renal failure. Nefrologia. 2011; 31:677–682.
- Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age and body size. Clin J Am Soc Nephrol. 2010;5:1003–1009.
- Pöge U, Gerhardt T, Stoffel-Wagner B, Sauerbruch T, Woitas RP. Validation of the CKD-EPI formula in patients after renal transplantation. Nephrol Dial Transplant. 2011;26:4104–4108.