Abstract
Objective: The objective of this study is to evaluate the effect and mechanism of aging on iodinated-contrast-media-induced nephropathy in male rats. Methods: Twenty-four healthy male rats were initially divided into 12-month-old and 24-month-old age groups (adult and older age groups, respectively; n = 12/group); subsequently, each age group was randomly divided into saline control (NS) and contrast media (CM) groups (n = 6/group). CM (76% diatrizoate, 10 mL/kg b.w.) was given through the caudal vein. Urinary creatinine (Ucr) and serum creatinine (Scr) were detected by an automatic biochemical analyzer. The activities of renal malondialdehyde (MDA), superoxide dismutase (SOD), angiotensin-converting enzyme (ACE), angiotensin II (Ang II), and reduced form of nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) were determined by spectrophotometric assays with commercially available kits according to the manufacturers’ protocols. Renal histological changes were observed by hematoxylin and eosin staining and scored semiquantitatively. Results: In diatrizoate-injected aged rats, Scr, the activities of ACE, Ang II, MDA, and NADPH oxidase in renal tissues were significantly increased (p < 0.01). The histologic scores were higher in the aged animals with CM treatment than those of control or adult rats (p < 0.01). There was an increasing trend but no significant statistical difference in renal ACE, Ang II, MDA, and NADPH oxidase or histologic scores in adult CM-injected rats compared with control animals (p > 0.05). Conclusions: Older age is an aggravating factor of iodinated-contrast-media-induced nephropathy in male rats. Oxidative stress and the renin–angiotensin system (RAS) may play an important role in nephrotoxicity induced by iodinated contrast media, especially in aged male rats.
INTRODUCTION
Contrast-induced nephropathy (CIN) is a condition in which an impairment in renal function (an increase in serum creatinine by more than 25% or 44 μmol/L) occurs within 3 days following the intravascular administration of a contrast medium in the absence of an alternative etiology.Citation1 Accounting for 10% of acute renal failure in hospitalized patients,Citation2 it is a serious clinical problem associated with increased morbidity and mortality, extended length of hospital stay, and increased health care costs.Citation3
The present studies and guidelines show that older age is a risk factor for the development of CIN,Citation1,4–7 but the exact pathogenesis of CIN in the elderly is not clearly understood. Recently, studies have shown that renal ischemia, direct toxicity to renal tubular epithelium cells, oxidative stress, and tubular obstruction are considerably linked to the pathogenesis of CIN.Citation8,9 Changes in renal hemodynamics are implicated in animal models due to the effects of contrast media on the action of many substances, including dopamine-1, adenosine, angiotensin II (Ang II), nitric oxide, and endothelin.Citation10–13 Our previous studies have indicated that serum ACE is a sensitive parameter for the early assessment of CIN, and renal Ang II might play a role in renal tubular cell apoptosis induced by contrast media.Citation14,15 However, local ACE and Ang II activity in renal tissue after contrast media administration remains unknown, especially in older age.
NADPH oxidase is a major regulatory enzyme for the production of reactive oxygen species (ROS) in cells, and it can be used as an important indicator of ROS status, and as a marker of renal tubular epithelial cell and vascular endothelial cell damage. When NADPH oxidase expression and activity increase, the large numbers of generated ROS promote oxidative stress.Citation16,17 Emerging data indicate that the expression level of NADPH correlates well with the progression of age-related chronic diseases, including obesity and hypertension.Citation18 The accumulated NADPH in aging renal tissue may enhance the susceptibility of the aging kidney to toxic substances.Citation4 However, the exact role of NADPH oxidase in the pathogenesis of CIN, especially in the aged kidney, remains unclear. Therefore, the aim of our study is to evaluate the effect and mechanism of age on iodinated contrast media-induced nephropathy in male rats.
MATERIALS AND METHODS
Reagents and Animals
The iodinated contrast medium was 76% diatrizoate meglumine (Xinyi Pharmaceutical Co., Ltd, Shanghai, China), diluted to 300 mg I/mL with distilled water. The ACE and Ang II assay kits were purchased from the Naval General Hospital (Beijing, China); malondialdehyde (MDA) and superoxide dismutase (SOD) assay kits were purchased from Jiancheng Bioengineering Institute (Nanjing, China); the NADPH oxidase assay kit was purchased from Genmed Scientifics Inc. (Shanghai, China); the bicinchoninic acid (BCA) assay kit was purchased from Biyuntian Biotechnological Co., Ltd (Jiangsu, China); and the immunohistochemical secondary antibody kit was purchased from Jingmei Biotechnological Co., Ltd (Shenzhen, China).
Twenty-four healthy male Sprague-Dawley rats with a body weight range of 230–690 g were purchased from the Second Xiangya Hospital Animal Center, China. According to the age category, they were first divided into 12 month and 24 month age groups (n = 12/group), and then each age group was randomly divided into saline (NS) control group and contrast media (CM) group, including four sub-groups (n = 6/group), named adult NS control group (AdNS), adult contrast media group (AdCM), aged NS control group (AgNS), and aged contrast media group (AgCM). All experiments were approved by the Medical Science Animal Care Committee of the Central South University.
Experimental Protocol
After water deprivation for 24 h, all CM groups were injected with 10 mL/kg b.w. of 76% diatrizoate through the caudal vein in 2 min; at the same time, the other two control groups were injected with similar volumes of saline. Urine samples before and 48 h after administration were collected to determine urinary creatinine (UCr) by the picric acid method. Blood samples were obtained before and 48 h after contrast media injection to evaluate the level of serum creatinine (Scr) and total cholesterol (TC) by an automatic biochemical analyzer (Hitachi 7170A, Japan). After the blood samples were collected, all rats were sacrificed by cervical dislocation.
Renal Histopathological Examination
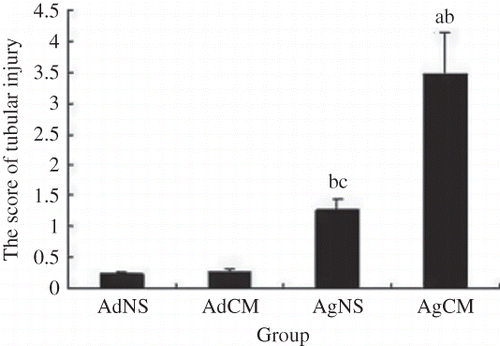
The right kidney was fixed with 10% buffered formalin and embedded in paraffin. The kidney samples were sectioned at four microns and stained by hematoxylin and eosin and Masson staining. Histological changes were observed and scored semiquantitatively. A minimum of 10 fields at ×200 magnification were assessed and graded in each biopsy. For tubular injury, the following score was used: 0 = no tubular injury; 1 = <25% of tubules injured; 2 = from 25 to 50% of tubules injured; 3 = from 51 to 75% of tubules injured; 4 = more than 76% of tubules injured.Citation19
Measurements of Renal MDA Levels, SOD, ACE, Ang II, and NADPH Oxidase Activities
The MDA, SOD, ACE, Ang II, and NADPH oxidase activities in the renal tissue were measured using commercial kits. In brief, 200 mg renal cortex was washed in ice-cold saline in tubes, cut into small pieces, and homogenized with ice-cold saline homogenization buffer at a ratio of 1:9 (v:w). The homogenate was centrifuged at 2000 rpm for 20 min at 4°C. The supernatant was separated and analyzed for total protein, which was followed by spectrophotometric determination of renal MDA level and SOD, ACE, Ang II, and NADPH oxidase activities using commercially available kits, according to the manufacturers’ protocol. The protein concentration of the homogenate was determined by the BCA assay method.
Statistical Analysis
All statistical analyses were carried out using SPSS for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA). All the data are expressed as mean values ± standard deviation (). For comparison between any two groups, group t-test was performed. Pearson’s correlation was used for analysis. p-Value <0.05 was considered statistically significant.
RESULTS
Changes of Scr and Ccr in Different Groups
Body weight, Scr, serum TC and Ccr values were identified among the four groups at the baseline. The serum TC values of the aged rats were higher than those of the adult animals (p < 0.05). In CM-administered rats, a trend toward higher values of Scr accompanied by reduced Ccr values was observed when compared with control animals, which was significantly higher (p < 0.01). The aged rats showed an evident increase in Scr by >25% from baseline (p < 0.01) and a decline in Ccr (p < 0.05) after CM administration; however, the increase in Scr in CM-injected adult animals was <25% from baseline (p < 0.05) with no significant difference observed in Ccr ().
Table 1. Changes of Scr and Ccr in different groups.
RAS and Oxidative Stress Parameters
As shown in and , a decline in kidney ACE and Ang II activities was observed in adult control rats, compared to control animals in the older age group (p < 0.05). When compared with the control animals or CM-injected adult rats, the activities of kidney ACE, Ang II, MDA, and NADPH oxidase were significantly increased in CM-administered aged rats (p < 0.01), and this was accompanied by reduced SOD (p < 0.01). In adult rats, no significant difference of kidney MDA and ACE activity was observed between control and CM animals (p > 0.05).
Table 2. Comparison of intrarenal ACE and Ag II activity in different groups.
Table 3. Comparison of intrarenal ROS-related parameters in different groups.
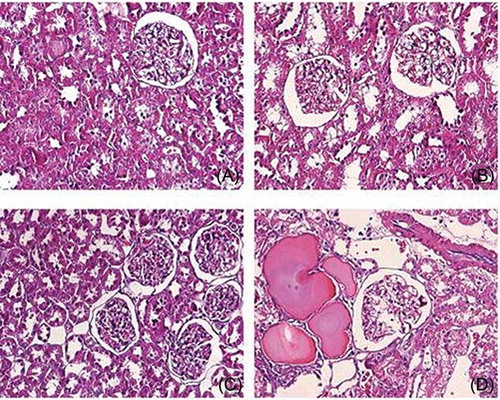
Renal Histomorphology
As shown in , in CM-injected aged rats, vacuolar degeneration of tubular epithelial cells, tubular dilation, protein cast, loss of tubular brush border, increased epithelial cell shedding, and necrosis of partial tubular epithelial cells were observed, in contrast to aged control animals. However, in CM-injected adult rats, mild swelling of renal interstitium and vacuolar degeneration of focal tubular epithelial cells was observed. Semiquantitative analysis showed that the scores of tubular injury in CM-injected aged rats were significantly higher compared to control animals or CM-injected adult rats (p < 0.01); No significant differences in the scores of tubular injury were observed between adult control and CM-injected animals (p > 0.05, ).
Correlation Analysis
The study showed that the activities of ACE, Ang II, MDA, and NADPH oxidase in renal tissue were positively correlated to Scr (γ = 0.803, γ = 0.834, γ = 0.812, γ = 0.865; p < 0.05, respectively) in aged rats.
DISCUSSION
Clinical studies have showed that older age is a risk factor for the development of CIN,Citation1,4–7 but the exact pathogenesis of CIN in the elderly is not clear. Considering the susceptibility of male animals for acute kidney injury,Citation20,21 we evaluated the effect and mechanism of aging on iodinated contrast media-induced nephropathy in male rats. The results indicated that 76% diatrizoate injection could result in typical kidney injury of CIN model, and older age might serve as an aggravating factor of iodinated CIN in male rats.Oxidative stress and RAS may play an important role in nephrotoxicity induced by iodinated contrast media, especially in aged male rats.
The mechanism of increased susceptibility to nephrotoxins in aging is incompletely understood and probably involves an age-dependent increase in ROS generation, decrease in renal reserve capacity, arterial sclerosis, renal blood flow changes, poor regulation of body function, decreased resistance, high sensitivity to risk factors, etc.Citation22,23 Previous studies have showed that hypercholesterolemia aggravates radiocontrast nephrotoxicity in rats.Citation24,25 In our study, the serum total cholesterol levels of aged rats were higher than those of the adult animals at baseline, which might contribute to the increased susceptibility of CIN in aged rats.
The role of RAS in the increased susceptibility to nephrotoxins in aging is complex. Recent studies have showed that RAS activity is decreased in aging-related chronic disease,Citation4,26 but increased after acute ischemia in aged rats.Citation27 Our data indicate that ACE and Ang II activities in renal tissue of aged rats decrease, in comparison to adult animals; this is consistent with previous research results,Citation4,26 indicating that the role of RAS in kidney disease could be context-dependent, and in aged rats, decreased activities of kidney ACE and Ang II may account for the susceptibility to CIN, which could be different from the mechanism in acute kidney injury. Further studies are necessary for ascertaining the exact role of RAS in kidney.
Recent studies indicate that the pathogenesis of CIN is related to increased kidney ROS.Citation1,17,18,28 NADPH oxidases are prime candidates as sources of ROS and can be used as important indicators of ROS status.Citation29 Oxidative stress is hypothesized to play a role in aging and age-related disorders, such as hypertension. Sónia Simão et al.Citation18 found that the expression of some oxidant and antioxidant enzymes was upregulated in old rats. The study of Akbar Ahmada et al.Citation30 showed that apocynin, a NADPH oxidase inhibitor, attenuated the degree of CIN in diabetic rats. However, the role of NADPH oxidase in the development of CIN, is not completely understood, especially in the elderly. Our study demonstrates that diatrizoate significantly increases MDA and NADPH oxidase activity in renal tissue and induced CIN model in aged rats; however, no obvious renal injury and significantly increased NADPH oxidase activity induced by diatrizoate in adult animals were observed. The correlation analysis shows that renal MDA and NADPH oxidase were positively correlated with Scr in CM-injected aged rats. It suggests that oxidative stress and NADPH oxidase may play a role in the pathogenesis of CIN induced by diatrizoate in the elderly.
Some studies have showed that disturbances of renal hemodynamics are one of the most important mechanisms for the development of CIN, which is mediated by RAS.Citation13,15,31 Clinical evidence has indicated that captopril (angiotensin-converting enzyme inhibitor, ACEI) reduces elevated Ang II and prevents CIN in diabetic patients.Citation31 Withholding ACEI or angiotensin receptor blocker 24 h before coronary angiography does not seem to influence the incidence of CIN in stable patients with chronic kidney disease stages 3–4.Citation13 Angiotensin receptor blockers prevent acute renal failure and delay the process of chronic kidney disease.Citation13,32 Our previous studies showed that serum and urinary ACE activities were increased 24 h–48 h after contrast media administration.Citation14 The renal Ang II levels in contrast media groups were higher than control groups and telmisartan protected renal tissue from nephrotoxicity induced by contrast media in glycerin-treated rats.Citation15 However, the effect of contrast media on Ang II of aged kidney tissue and the role of RAS on elderly CIN are unclear. Our study indicates that renal ACE and Ang II activities in aged rats increase significantly after contrast media administration. It further supports an important role for increased RAS in the pathogenesis of CIN, and indicates that it might be a contributory factor in the development of CIN in aged rats.
In conclusion, our study suggests that older age is an aggravating factor of iodinated contrast-induced nephropathy in male rats. Oxidative stress and RAS may play important roles in nephrotoxicity induced by iodinated contrast media in aged male rats. Further study is needed to clarify the exact nature of oxidative stress and RAS in the pathogenesis of CIN.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The work was supported by grants from the Scientific Foundation of Hunan Province, China (2010FJ6008 and 2008JT3005).
REFERENCES
- Stacul F, van der Molen AJ, Reimer P, . Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527–2541.
- Maeder M, Klein M, Fehr T, Rickli H. Contrast nephropathy: review focusing on prevention. J Am Coll Cardiol. 2004;44:1763–1771.
- Toprak O, Cirit M. Risk factors for contrast-induced nephropathy. Kidney Blood Press Res. 2006;29:84–93.
- Jerkić M, Vojvodić S, López-Novoa JM. The mechanism of increased renal susceptibility to toxic substances in the elderly. Part I. The role of increased vasoconstriction. Int Urol Nephrol. 2001;32:539–547.
- Cronin RE. Contrast-induced nephropathy: pathogenesis and prevention. Pediatr Nephrol. 2010;25:191–204.
- Vercellino M, Bezante GP, Balbi M. Contrast medium induced nephropathy: new insights into prevention and risk management. Cardiovasc Hematol Agents Med Chem. 2009;7:166–180.
- Carrillo-Alvira F, Rivera-Bermúdez CG, Jiménez-Velázquez IZ, Ramos-Romey C, González-Concepción JJ. Contrast associated nephropathy in the elderly. Bol Asoc Med P R. 2008;100:25–27.
- Katzberg RW. Contrast medium-induced nephrotoxicity: which pathway? Radiology. 2005;235:752–755.
- Maeder M, Klein M, Fehr T, Rickli H. Contrast nephropathy: review focusing on prevention. J Am Coll Cardiol. 2004;44:1763–1771.
- Duan SB, Liu FY, Luo JA, . Nephrotoxicity of high- and low-osmolar contrast media. The protective role of amlodipine in a rat model. Acta Radiol. 2000;41:503–507.
- Russo D, Minutolo R, Cianciaruso B, Memoli B, Conte G, De Nicola L. Early effects of contrast media on renal hemodynamics and tubular function in chronic renal failure. J Am Soc Nephrol. 1995;6:1451–1458.
- Bakris GL, Lass NA, Glock D. Renal hemodynamics in radiocontrast medium-induced renal dysfunction: a role for dopamine-1 receptors. Kidney Int. 1999;56:206–210.
- Rosenstock JL, Bruno R, Kim JK, . The effect of withdrawal of ACE inhibitors or angiotensin receptor blockers prior to coronary angiography on the incidence of contrast-induced nephropathy. Int Urol Nephrol. 2008;40:749–755.
- Duan SB, Wu HW, Luo JA, Liu FY. Assessment of renal function in the early stages of nephrotoxicity induced by iodinated contrast media. Nephron. 1999;83:122–125.
- Duan SB, Wang YH, Liu FY, . The protective role of telmisartan against nephrotoxicity induced by X-ray contrast media in rat model. Acta Radiol. 2009;50:754–759.
- Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal. 2009;11:2607–2619.
- Heyman SN, Rosen S, Khamaisi M, Idée JM, Rosenberger C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol. 2010;45:188–195.
- Simão S, Gomes P, Pinto V, . Age-related changes in renal expression of oxidant and antioxidant enzymes and oxidative stress markers in male SHR and WKY rats. Exp Gerontol. 2011;46:468–474.
- Capasso G, Di Gennaro CI, Della Raqione F, . In vivo effect of the natural antioxidant hydroxytyrosol on cyclosporine nephrotoxicity in rats. Nephrol Dial Transplant. 2008;23:1186–1195.
- Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem. 2004;279:52282–52292.
- Golab F, Kadkhodaee M, Xu J, Soleimani M. Male susceptibility to hepatic damage in acute uremia in rats. Urology. 2011;78(232):e1–e6.
- Pucelikova T, Dangas G, Mehran R. Contrast-induced nephropathy. Catheter Cardiovasc Interv. 2008;71:62–72.
- Presta P, Lucisano G, Fuiano L, Fuiano G. The kidney and the elderly: why does the risk increase? Int Urol Nephrol. 2012;44:625–632.
- Andrade L, Campos SB, Seguro AC. Hypercholesterolemia aggravates radiocontrast nephrotoxicity: protective role of L-arginine. Kidney Int. 1998;53:1736–1742.
- Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473.
- Corman B, Barrault MB, Klingler C, . Renin gene expression in the aging kidney: effect of sodium restriction. Mech Ageing Dev. 1995;84:1–13.
- Lü X, Li X, Li L, Li L, Li C, Wang H. Variation of intrarenal angiotensin II and angiotensin II receptors by acute renal ischemia in the aged rat. Ren Fail. 1996;18:19–29.
- Wong PC, Li Z, Guo J, Zhang A. Pathophysiology of contrast-induced nephropathy. Int J Cardiol. 2012;158:186–192.
- Khanna AK, Pieper GM. NADPH oxidase subunits (NOX-1, p22phox, Rac-1) and tacrolimus-induced nephrotoxicity in a rat renal transplant model. Nephrol Dial Transplant. 2007;22:376–385.
- Ahmad A, Mondello S, Di Paola R, . Protective effect of apocynin, a NADPH-oxidase inhibitor, against contrast-induced nephropathy in the diabetic rats: a comparison with n-acetylcysteine. Eur J Pharmacol. 2012;674:397–406.
- Gupta RK, Kapoor A, Tewari S, Sinha N, Sharma RK. Captopril for prevention of contrast-induced nephropathy in diabetic patients: a randomised study. Indian Heart J. 1999;51:521–526.
- Shoda J, Kanno Y, Suzuki H. A five-year comparison of the renal protective effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with non-diabetic nephropathy. Intern Med. 2006;45:193–198.