Abstract
We investigated the protective effect and mechanism of neutrophil gelatinase-associated lipocalin (NGAL) on rats ischemia/reperfusion (I/R) renal injury. Eighteen Sprague-Dawley male rats were randomly divided into three groups. Control group (n = 6) suffered left unilateral nephrectomy, I/R + NS (normal saline) (n = 6) and I/R + NGAL (n = 6) group were subjected to 45 min right renal ischemia/24 h reperfusion after left unilateral nephrectomy. Serum creatinine (Scr) and blood urea nitrogen (Bun) were measured on automatic biochemistry analyzer; kidney sections were stained with hematoxylin-eosin; terminal dUTP nick-labeling method was used to examine the apoptosis of tubular epithelial cells; Cleaved caspase-3 and Bax protein expression were detected by immunohistochemistry and Western Blot; real-time polymerase chain reaction was used to detect the expression of Bax mRNA. Rats with NGAL displayed an attenuated renal damage and a decreased number of tubular epithelial cell apoptosis compared to the I/R + NS group (Scr 63.400 ± 11.908 vs. 121.857 ± 17.151 μmol/L, Bun 14.840 ± 2.868 vs. 28.557 ± 6.434 mmol/L, apoptosis cell number 7.800 ± 1.924 vs. 15.400 ± 3.049/high power field (HPF), p < 0.05), the values were lower in the control group (24.000 ± 3.829 μmol/L, 5.814 ± 1.961 mmol/L, 1.800 ± 0.837/HPF, p < 0.05) compared to two groups above; NGAL-treated rats showed down-regulated Cleaved caspase-3 protein (0.284 ± 0.066 vs. 0.409 ± 0.073, p < 0.05), Bax protein (0.346 ± 0.055 vs. 0.443 ± 0.041, p < 0.05), Bax mRNA (1.423 ± 0.187 vs. 2.550 ± 0.217, p < 0.05) compared to I/R + NS group, but the values were higher in both of the two groups than those in the control group (Cleaved caspase-3 protein 0.104 ± 0.029, Bax protein 0.155 ± 0.027, Bax mRNA 1.000 ± 0.000, p < 0.05). We supposed that exogenous NGAL can inhibit the activation of caspase-3, reduce the expression of Bax, and thus reduce renal tubular cell apoptosis and protect renal function in I/R injury rats.
INTRODUCTION
Ischemia and toxins are the most important causes of acute kidney injury (AKI).Citation1 The prevalence of AKI in all hospitalized patients is 5–35%, which is associated with a two- to five-fold increased mortality risk.Citation2 Neutrophil gelatinase-associated lipocalin (NGAL, also known as Lipocalin 2) has been shown to be dramatically induced early in ischemic renal injury (IRI) by use of cDNA microarrays.Citation3 The small 25kDa peptide is a secret protein, initially found in human neutrophilsCitation4,5 with its mouse analog 24p3 belonging to the lipocalin superfamily.Citation6,7 Subsequently, it is also been found in other types of cells, including tubular cells of the kidney, which suffered various injuries.Citation8 As an acute phase protein, it is currently one of the interesting proteins involved in the phase of AKI. Recently experimented evidence indicates that exogenous NGAL could ameliorate the morphologic and functional consequences in a murine model of renal IRI and reduce the apoptotic tubule cell death,Citation9 which has led to the hypothesis that NGAL may prevent tubular injury. On the basis of recent studies, we know that circulating NGAL was filtrated by glomerular and subsequent uptake by the proximal tubule,Citation10 but the consequent protective mechanism was not well understood and this understanding will greatly advance further studies. Apoptosis has recently emerged as the important form of tubule cell death following IRI.Citation11 Cell death signaling pathways include endogenous apoptosis pathways mediated by mitochondria and exogenous apoptotic pathways mediated by death receptor.Citation12,13 The caspases family and Bcl-2 family are considered to play an important role in mitochondrial apoptotic pathways.Citation12,14 Furthermore, caspase-3 is the downstream member of the caspases family, activated by the initiators (caspase-8, caspase-9) and converted into the active form Cleaved caspase-3 (Cc3), which plays an important part in apoptosis.Citation14,15 Bax is considered the representative pro-apoptotic member of Bcl-2 family.Citation12 L. Gong et al have previously published that NGAL attenuated renal injury and inhibited renal tubular epithelial cell apoptosis via Bcl-2/Bax signaling pathways.Citation16 According to the aforementioned reports, it was hypothesized that NGAL may be protective for renal ischemia/reperfusion injury, and this effect may be correlated with reducing renal tubular epithelial cell apoptosis. In order to elucidate the hypothesis, we study the effect of NGAL to the renal function, expression of Cleaved caspase-3, and pro-apoptotic Bax protein in an ischemia/reperfusion rats model.
MATERIALS AND METHODS
In vivo Experimental Procedure
Our study was approved by the Institutional Animal Care and Use Committee, and the procedures were performed according to the guidelines of the National Institute of Health.
Eighteen Sprague Dawley (Sino-British SIPPR/BK Lab. Animal Ltd., Co., Shanghai, China) male rats were randomly divided into the following three groups: control group (n = 6), ischemia/reperfusion [group (I/R + NS), n = 6] group, and I/R + NGAL [group(I/R + NGAL), n = 6] group.
Rats were fasted 12 h before operation, but was free to take water. Rats weighting 180–220 g were anesthetized with an intraperitoneal injection of 0.5 mL/100 g of 1% pentobarbital sodium (Shanghai, China). Then the left renal was removed, and the right renal pedicle was nipped by an artery clamp for 45 min. The same procedure was performed in the control group without the unilateral clamping process.
Before the operation, 50 μg of NGAL (Sino Biological Inc., Beijing, China) was dissolved in 2 mL of sterile water and administered intravenously into rats via tail-vein injections for four times, including 30 min before renal pedicle clamping, during renal pedicle clamping, and 30 min and 60 min after renal pedicle clamping; the other groups were injected the same amount of normal saline.
The Collection of Samples
Blood sample: After 24 h of reperfusion, blood samples were harvested from the inferior vena cava and centrifuged according to the following parameters: 4°C, 2500 rpm/min, and 15 min. Then the supernatant was collected and stored at −80°C.
Tissue sample: The kidney was cut along the coronal section, and one portion was fixed in 4% paraformaldehyde, while the other was snap-frozen in liquid nitrogen, and stored at −80°C.
Renal Function and Histopathology
The collected supernatant was measured in an automatic biochemistry (SIMENS Advia 2400) analyzer for serum creatinine (Scr) and blood urea nitrogen (Bun).
After fixing in 4% paraformaldehyde, the kidneys were embedded in paraffin. The tissues were sectioned at 3 μm for hematoxylin and eosin staining.
TUNEL Staining
The apoptosis was detected using an in situ cell apoptosis detection kit I (Boster, Wuhan, China). The permeability of cell membranes was increased by incubating the sections in 50 μL of proteinase K (1:200) at 37°C for 10 min. The mixture of Terminal deoxynucleotidyl Transferase (TbT) and digoxigenin labeled dUTP (Did-dUTP) was added to the sections for 2 h at 37°C. The biotin signed anti-digoxigenin antibody was applied for 30 min at 37°C. Sections were washed with 0.01M TBS for three times and combined with strep avidin-biotin complex (SABC), then dyed with DAB Staining Kit (Boster, Wuhan, China). For each specimen, cells with positive nuclei staining from 10 random microscopic fields (400×) were counted. Data were expressed as positive cell count, which is the mean of cells positive for apoptotic nuclei per microscopic field.
Immunohistochemistry
Paraffin sections were deparaffinized in xylene and rehydrated gradually through a series of graded alcohols. Following this, the sections were subjected to an antigen retrieval procedure by heating the sections in a microwave oven (700 W) in 0.01M sodium citrate buffer (pH 6.0) for 8 min for two times. After cooling for 30 min at room temperature (RT), the sections were washed in 0.01M PBS for three times, after which they were treated in darkness for 15 min with 3% hydrogen peroxide to reduced endogenous peroxidase. Then the sections were washed in 0.01M PBS for three times, and incubated with blocking solution (5% normal goat serum in 0.01M PBS) for 1 h at RT to reduce nonspecific binding. Following this, the sections were incubated with rabbit polyclonal antibody for Cleaved caspase-3 (1:200, Cell Signaling Technology, USA) and rabbit polyclonal antibody for Bax (1:50, Santa Cruz Biotechnology, CA, USA) for 12–18 h at 4°C, and the ready to use goat anti-rabbit secondary antibody was obtained from Maixin-Bio (Fuzhou, China). The immunohistochemistry data were expressed as the proportion of positive cell area in the total area. The analysis was carried using an Axioplan 2 imaging microscopic image analysis system (Axioplan 2 imaging KS400, ZEISS Co., Oberkochen, Germany). For each specimen, two random microscopic fields (400×) were analyzed (12 fields/group).
Western Blot Analysis
The kidney samples (60 mg) were pulverized in liquid nitrogen and then lysed in cell lysis buffer for Western and IP (Beyotime, Shanghai, China). Then, the lysate was centrifuged at 10,000 × g for 5 min and the supernatant was used to extract protein according to the manufacturer’s protocol (Nuclear and Cytoplasmic Protein Extraction Kit, Beyotime, Shanghai, China). Protein concentration was measured using the BCA protein assay kit (Pirece, Rockford, IL, USA). Samples of 45 μg of protein were loaded onto SDS-polyacrylamide gel for electrophoreses as follows: 4.5% spacer gel at 120 V for the coloring matter closing to the top of separation gel, 10% separation gel at 160 V until the bromophenol blue running to the bottom of gel. The separated protein was transferred to the PVDF membranes (Millipore, Billerica, MA, USA) at 45 V for 1.5 h. Then the members were blocked by 5% skimmed milk for 1.5 h followed by incubation with the primary anti-Cc3 (1:1000) and anti-Bax (1:300) antibodies through the night at 4°C. The samples were treated with anti-rabbit horseradish peroxidase-labeled secondary antibody and visualized by using a chemiluminescent substrate (Fast western blot kits, Pierce ECL, USA) and observed with an X-ray film (Kodak, Tokyo, Japan). The images were analyzed and quantified using Image Processing and Analysis in Java software (Image J 1.44p, National Institutes of Health, Bethesda, MD, USA).
RNA Isolation and qRT-PCR
Frozen kidney samples (100 mg) were ground with liquid nitrogen and then added into Trizol (Invitrogen, Carlsbad, CA, USA) to extract total RNA of tissue. RNA concentration was measured using SmartSpec Plus spectrophotometer (Bio-Rad, Hercules, CA, USA). Then 500 ng of total RNA was reversely transcribed into first-strand cDNA using PrimeScript RT master mix (Takara, Dalian, China). We performed real-time PCR to quantify Bax mRNA expression in a continuous fluorescence detector (Bio-Rad, USA) with a SYBR premix ex taq II (Takara, Dalian, China). The amplification reaction volume was 20 μL, which comprised 10 μL of SYBR Premix Ex Taq II, 4 μL of primes, 2 μL of cDNA and 4 μL of H2O. The PCR conditions included denaturation at 95°C for 5 min and followed 40 cycles consisting of 95°C for 15 s and 60°C for 1 min.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal control. The specific primers were synthesized by Takara (Dalian, China) as follows:
Bax forward 5′-AGACACCTGAGCTGACCTTGGA-3′, Bax reverse 5′-TTGAAGTTGCCATCAGCAAACA-3′, GAPDH forward 5′-GGCACAGTCAAGGCTGAGA ATG-3′, GAPDH reverse 5′-ATGGTGGTGAAGACGC CAGTA-3′. Then, the data were analyzed using the 2-ΔΔCTCitation17 method. As ΔCT = CT (target gene) - CT (internal control) and ΔΔCT = ΔCT (treatment groups) - ΔCT (control group).
Statistics
All data were expressed as mean ± SD. For the evaluation of results, ANOVA and least significant difference test (LSD) were used. The results were considered statistically significant if p < 0.05.
RESULTS
Effects of NGAL on Rats Histology and Renal Function
Analysis of the routine HE-stained sections of the I/R + NS group showed that there was evident loss of brush borders, flattening, vacuolation and cast, whereas NGAL-treated rats attenuated the renal tubular injuries, and the control group displayed normal histology (A–C). Scr and Bun were used to test the change of renal function. The Scr and Bun levels () were significantly elevated in the I/R + NGAL and I/R + NS group at 24 h after reperfusion compared with the control group (Scr 24.000 ± 3.829 μmol/L, Bun 5.814 ± 1.961 mmol/L). However, rats with NGAL had preserved renal function compared with rats in IRI (Scr 63.400 ± 11.908 vs. 121.857 ± 17.151 μmol/L, Bun 14.840 ± 2.868 vs. 28.557 ± 6.434 mmol/L, p < 0.05).
Figure 1. Sections of renal tissue were stained with H&E at 24 h after reperfusion. Rats with NGAL displayed an attenuated histopathologic response compared to I/R + NS rat. (A) Control group, (B) I/R + NGAL group, (C) I/R + NS group. Original magnification, ×400. Scale bars: 20 μm.
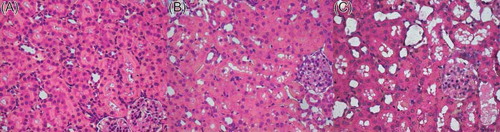
Table 1. Serum creatinine and blood urea nitrogen (mean ± SD; n = 6).
NGAL Reduced Apoptosis Induced by I/R Renal Injury
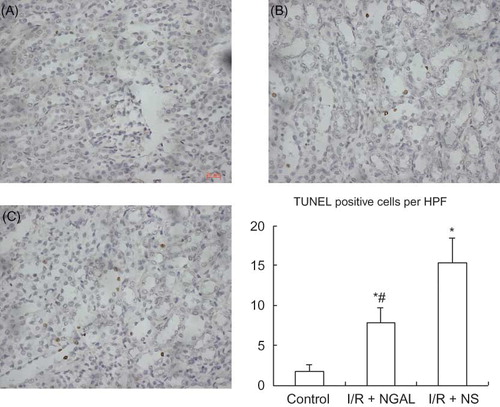
The TUNEL method was used to evaluate cellular apoptosis induced by ischemic and reperfusion. TUNEL-positive cells mainly existed in renal tubular epithelial cells, hardly expressed in glomerulus and renal interstitium. The control group displayed a minimal incidence of apoptosis (A) (1.800 ± 0.837/HPF). After ischemia/reperfusion, apoptosis occurred in more and more epithelial cells; however, after treatment of rats with NGAL, apoptosis in epithelial cells decreased remarkably (B–C) (7.800 ± 1.924 vs. 15.400 ± 3.049/HPF, p < 0.05).
Figure 2. Photomicrographs of TUNEL-stained kidney sections of rat. Control group (A) displayed a minimal incidence of apoptosis. TUNEL staining showed dramatically fewer apoptotic nuclei in NGAL-treated rat (B) compared to the I/R + NS group (C). Original magnification, ×400. Scale bars: 20 μm. The number of TUNEL-positive cells was for 10 images/slide and expresses as positive cells per high power field (HPF).Note: *p < 0.05 versus control group, #p < 0.05 versus I/R + NS group. Data are mean ± standard deviation (SD) for n = 6 animals per group.
NGAL Reduced the Expressions of Cc3 and Bax
The protein expression of Cleaved caspase-3 was a marker of cell apoptosis.Citation18 Note the immunoreactivity in the nucleus of renal tubular epithelial cells but not in other segments of the nephron. Cc3 accumulation increased significantly in the kidney in the I/R + NGAL and I/R + NS group compared with the control group (A and ) (0.608 ± 0.148). But NGAL-treated rats prevented elevation of the positive expression ration of Cc3 than that in the I/R + NS group (B–C and ) (3.171 ± 0.321 vs. 7.291 ± 1.059, p < 0.05). The same results were seen in Western blot analysis (A), and the value was lower in the control group (C) (0.104 ± 0.029) compared to the I/R + NGAL and I/R + NS group, whereas the NGAL-treated rats showed down-regulated Cc3 protein compared to the I/R + NS group (C) (0.284 ± 0.066 vs. 0.409 ± 0.073, p < 0.05). In our research, we found that the expression of Bax was limited to the cytoplasm of renal tubular epithelial cells, which was according to former report.Citation19 The control group had little staining for Bax (D). The ration of Bax positive expression increased significantly in I/R + NGAL and I/R + NS group than that in the control group () (3.058 ± 0.601). Statistically, the expression of Bax was lower in NGAL-treated rats than that in the I/R + NS group (E–F and ) (9.038 ± 2.451 vs. 24.862 ± 5.036, p < 0.05). This was in good accordance with the results from the Western blot (B and D) (I/R + NGAL 0.346 ± 0.055 vs. I/R + NS 0.443 ± 0.041, p < 0.05), and both the groups were higher than the control group (0.155 ± 0.027). The increased expression of protein Bax may play some role in the IRI; however, exogenous NGAL may control this process.
Figure 3. Immunohistochemical staining of Cleaved caspase-3 and Bax in kidney tissue at 24 h after reperfusion. (A–C) Cleaved caspase-3 located mainly in nucleus of renal tubular epithelial cells. (D–F) Bax existed mainly in cytoplasm of renal tubular epithelial cells. (A) and (D); Control group, (B) and (E); I/R + NGAL group, (C) and (F); I/R + NS group. Original magnification, ×400. Scale bars: 20 μm.
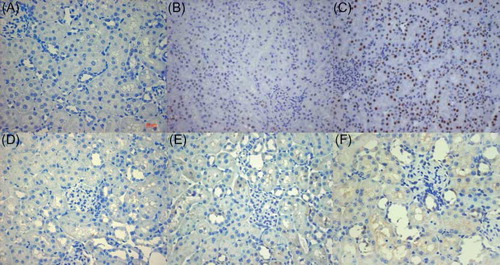
Figure 4. Western blot analysis of Cleaved caspase-3 and Bax in renal tubular epithelial cells. Representative immunoblots of Cleaved caspase-3 (A) and Bax (B). Densitometric quantification of Cleaved caspase-3 (C) and Bax (D). Note: *p < 0.05 versus control group, #p < 0.05 versus I/R + NS group. Data are mean ± standard deviation (SD) for n = 6 animals per group.
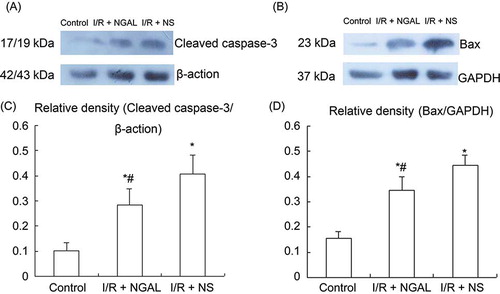
Figure 5. The mRNA expression of Bax gene in kidney of rat. Quantification of Bax mRNA normalized to GAPDH. NGAL-treated rat reduced the level of Bax mRNA compared to the I/R + NS group. Note: *p < 0.05 versus the control group, #p < 0.05 versus I/R + NS group. Data are mean ± standard deviation (SD) for n = 6 animals per group.
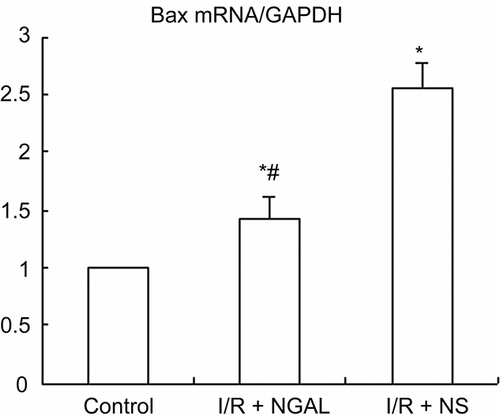
Table 2. Positive rations of Cleaved caspase-3 and Bax (mean ± SD; n = 12).
The Effects of NGAL on Bax mRNA
Regarding the level of mRNA, there was an evident increase of Bax mRNA expression in the I/R + NGAL and I/R + NS groups than that in the control group () (1.000 ± 0.000) at 24 h after reperfusion. However, NGAL-treated rats expressed less Bax mRNA than that in the I/R + NS group (1.423 ± 0.187 vs. 2.550 ± 0.217, p < 0.05).
DISCUSSION
NGAL is a member of a large family of lipocalins.Citation20 Initially, it was found in activated neutrophilsCitation8; however, researchers found that NGAL may also be expressed in other cells such as in stomach, liver, and epithelial tissue under certain conditionsCitation6,21 including renal tubular epithelial cells, which suffered various injuries.Citation8 Mishra et al. compared the protective action of exogenous NGAL on renal mice 1 h before ischemia, during ischemia, 1 h after ischemia, and found that the action could prevent renal injury each of the three times. It was indicated that NGAL could play an important protective role during 1 h before ischemia and 1 h after ischemia.Citation9 In our research, NGAL was administered intravenously into rat via tail-vein injections for four times, including 30 min before renal pedicle clamping, during renal pedicle clamping, 30 min and 60 min after renal pedicle clamping to study the protective effect and mechanism of NGAL on rats I/R renal injury. The results showed that exogenous NGAL could inhibit the rise of serum creatinine (Scr) and blood urea nitrogen (Bun), alleviate kidney injury, and reduce renal tubular epithelial cells apoptosis.
The intrinsic difference of cell apoptosis and necrosis is that apoptosis is a programmed cell death process. In animals, apoptosis is the predominant mode of cell death during development and tissue homeostasis.Citation18 A major biochemical change of apoptosis includes nuclear fragmentation and chromatin condensation with DNA fragmentation.Citation22 In this study, we used terminal deoxynucleotidyl transferase-mediated UTP nick end labeling (TUNEL) to detect apoptotic cell death. It turned out that apoptosis occurred mainly in renal tubular epithelial cells of renal outer medullary region, which corresponded to the result of Sutton, that outer the medullary region was the worst area after ischemic AKI.Citation23 The apoptosis of the I/R + NGAL group significantly reduced than that in I/R + NS group, and this was consistent with former reports. Mishra et al. found that exogenous NGAL alleviated renal injury of I/R mice and reduced apoptosis of renal tubular epithelial cells.Citation9,10 In vitro experiments also confirmed that exogenous NGAL could inhibit the apoptosis of HK-2 cells, which suffered hypoxia (1 h) and reoxygenation (24 h).Citation24 Thus, we assumed that NGAL may play a role in renal protection via inhibiting the apoptosis of renal tubular epithelial cells.
Our results of immunohistochemistry confirmed that the Cleaved caspase-3 (Cc3) mainly existed in the nucleus of renal tubular epithelial cells after ischemia (45 min) and reperfusion (24 h); the control group expressed only a little Cc3; the expression of Cc3 in I/R + NS group was elevated compared with the control group, and this correlated with the results of other studies.Citation18,25 After treatment with NGAL, rats showed down-regulated Cc3 protein compared with I/R + NS rats. Caspases are intracellular proteases family that regulate programmed cell death, and the activation of the caspase family is a key part in apoptosis.Citation14,15 Caspase-3 is an executioner of the family, is a downstream member, and it exists predominantly in cytoplasm in zymogen form without any stresses.Citation26,27 After activation by proteolysis of the upstream processors of effector caspases, it translocated to the nucleus to cleave many downstream substrates to induce apoptotic cells death.Citation14,15 We also used immunohistochemistry to observe the change of Bax expression after I/R renal injury. The results showed that Bax mainly located in the cytoplasm of renal tubular epithelial cells in the non-ischemic control group, but the level was lower, which is in accordance with another report.Citation19 After ischemia and reperfusion, the Bax in cytoplasm increased abundantly. However, the expression of Bax reduced evidently after treatment with NGAL compared with the I/R + NS group. The results of Western blot also confirmed that NGAL could reduce the expression of Bax evidently. We used the real-time PCR method to detect the change of Bax mRNA expression and found that NGAL-treated rats expressed less Bax mRNA than that in the I/R + NS group. One experiment in vitro also showed that exogenous NGAL could reduce the level of Bax/Bcl-2 mRNA in HK-2 cells, which suffered hypoxia and reoxygenation.Citation24 So we assumed that the renoprotection effect of NGAL was achieved mainly by inhibiting the activation of caspase-3, reducing the expression of Bax, and thus inhibiting cell apoptosis.
In conclusion, both Cleaved caspase-3 and Bax take part in the pathological physiology process of renal ischemia/reperfusion injury, exogenous NGAL can protect renal injury via inhibiting the activation of caspase-3 and reducing the expression of Bax. However, further experiments are needed to investigate how NGAL gives a signal to regulate the expression of caspase-3 and Bax. In our study, we administrated NGAL only in divided doses during the effective treatment window, and in future research, we should observe the therapeutic effect of single dose and multiple doses to provide more effective and exact therapy for AKI.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
This work was supported by ShangHai Municipal Human Resources and Social Security Bureau.
REFERENCES
- Sabbahy ME, Vaidya VS. Ischemic kidney injury and mechanisms of tissue repair. Wiley Interdiscip Rev Syst Biol Med. 2011;3(5):606–618.
- Kanbay M, Kasapoglu B, Perazella MA. Acute tubular necrosis and pre-renal acute kidney injury: utility of urine microscopy in their evaluation—a systematic review. Int Urol Nephrol. 2010;42:425–433.
- Devarajan P, Mishra J, Supavekin S, Patterson LT, Steven Potter S. Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery novel therapeutics. Mol Genet Metab. 2003;80(4):365–376.
- Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268(14):10425–10432.
- Kjeldsen L, Bainton DF, Sengeløv H, Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood. 1994;83(3):799–807.
- Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45(1):17–23.
- Tong Z, Wu X, Ovcharenko D, Zhu JX, Chen CS, Kehrer JP. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005;391(Pt2):441–448.
- Zhao C, Ozaeta P, Fishpaugh J, . Structural characterization of glycoprotein NGAL, an early predictive biomarker for acute kidney injury. Carbohydr Res. 2010;345:2252–2261.
- Mishra J, Mori K, Ma Q, . Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15:3073–3082.
- Mori K, Lee HT, Rapoport D, . Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–621.
- Dagher PC. Apoptosis in ischemic renal injury: roles of GTP depletion and p53. Kidney Int. 2004;66(2):506–509.
- Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122(Pt4):437–441.
- Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17(20):2481–2495.
- Takemoto K, Nagai T, Miyawaki A, Miura M. Spatio-temporal activation of caspase revealed by indicator that is insensitive to environmental effects. J Cell Biol. 2003;160(2):235–243.
- Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284(33):21777–21781.
- Gong L, Yu H, Zhuge Y, Yu Q. Neutrophil gelatinase-associated lipocalin protects renal tubular epithelial cell in ischemic/reperfusion injury rats via apoptosis-regulating proteins. Ren Fail. 2012;34(6):777–783.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTMethod. Methods. 2001;25(4):402–408.
- Raff U, Schneider R, Gambaryan S, . L-arginine does not affect renal morphology and cell survival in ischemic acute renal failure in rats. Nephron Physiol. 2005;101(3):39–50.
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37(3):299–310.
- Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318(Pt1):1–14.
- Friedl A, Stoesz SP, Buckley P, Gould MN. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J. 1999;31(7):433–441.
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257.
- Sutton TA. Alteration of microvascular permeability in acute kidney injury. Microvasc Res. 2009;77(1):4–7.
- Cui LY, Yang S, Protective ZJ. Effects of neutrophil gelatinase- associated lipocalin on hypoxia/reoxygenation injury of HK-2 cells. Transplant Proc. 2011;43(10):3622–3627.
- Betz B, Schneider R, Kress T, Schick MA, Wanner C, Sauvant C. Rosiglitazone affects nitric oxide synthases and improves renal outcome in a rat model of severe ischemia/reperfusion injury. PPAR Res. 2012;2012: 219319. Epub 2012 Feb 15.
- Faleiro L, Caspases LY. Disrupt the nuclear-cytoplasmic barrier. J Cell Biol. 2000;151(5):951–959.
- Zhivotovsky B, Samali A, Gahm A, Orrenius S. Caspases: their intracellular localization and translocation during apoptosis. Cell Death Differ. 1999;6(7):644–651.
