Abstract
Background: Upper gastrointestinal bleeding (UGIB) is a major cause of clinical bleeding among patients with end-stage renal disease (ESRD). This study aimed to investigate the association between mortality and UGIB in patients with uremia. Methods: From 2004 to 2010, a tertiary hospital-based retrospective cohort comprising 322 patients undergoing hemodialysis was investigated. All the patients were diagnosed with UGIB according to the International Classification of Diseases, 9th Revision (ICD-9) that included peptic ulcer bleeding, duodenal ulcer bleeding, and other symptoms. UGIB was required to be one of the first three discharge diagnoses. Rehospitalization within 3 days after discharge was regarded as the same course. Exclusion criteria were age <20 years, previous gastric resection or vagotomy, esophageal and gastric variceal bleeding, or gastric cancer within the first 2 years of the index hospitalization. Results: The all-cause in-hospital mortality rate of patients with UGIB undergoing hemodialysis was high, with the first-month mortality rate of 13.7%, sixth-month mortality rate of 26.7%, and first-year mortality rate of 27.0%. Using Cox regression models, we found that the high mortality rate of the UGIB group was significantly correlated with older age [adjusted hazard ratio (HR) = 1.02, 95% confidence interval (CI) = 1.01–1.04], female sex (adjusted HR = 1.62, 95% CI = 1.05–2.51), infection during hospitalization (adjusted HR = 1.85, 95% CI = 1.13–3.03), single episodic UGIB (adjusted HR = 2.00, 95% CI = 1.08–3.70), abnormal white blood cell (WBC) count (adjusted HR = 1.59, 95% CI = 1.03–2.45), and albumin level ≤3 g/dL (adjusted HR = 2.67, 95% CI = 1.51–4.72). Conclusion: In conclusion, patients with ESRD who are admitted with primary UGIB have a profoundly increased risk of all-cause in-hospital mortality during the follow-up period.
INTRODUCTION
Upper gastrointestinal bleeding (UGIB) encompasses a broad array of clinical scenarios in patients with uremia. In patients with renal failure, UGIB can vary from occult to massive hemorrhage and lead to higher morbidity and mortality rates than in the general population.Citation1,2 Early autopsy studies of patients who died of uremia revealed common gastrointestinal abnormalities, including edema, congestion, and ulcerations with hemorrhage.Citation3 Helicobacter pylori infection, cigarette smoking, use of nonsteroidal anti-inflammatory drugs (NSAIDs), and older age are well-established traditional risk factors for UGIB.Citation1,4 However, a recent study by Wong et al. found that peptic ulcer re-bleeding and mortality rates were not high in patients with H. pylori infection or those taking NSAIDs.Citation5 Another retrospective study showed that patients with end-stage renal disease (ESRD) and peptic ulcer bleeding had higher re-bleeding and mortality rates than patients not on dialysis.Citation6 A greater number of comorbidities were found to be the only significant predictor of mortality in multivariate analysis [odds ratio (OR) = 6.0, 95% confidence interval (CI) = 2.9–12.3; p = 0.001].Citation6 UGIB had been estimated to account for 3–7% of all deaths among patients with ESRD.Citation7
The diminishing incidences of gastric ulcer and duodenal ulcer diseases of 42–48% and 41–71%, respectively, between 1997 and 2006 in Taiwan may be attributed to early diagnosis and effective use of acid suppressive drugs.Citation8 However, data from the US Renal Data System showed that the rate of nonvariceal UGIB among patients undergoing dialysis had not decreased over the past 10 years.Citation9 The pathogenesis of UGIB in ESRD may be related to impaired hemostasis caused by intrinsic platelet abnormalities, impaired platelet–vessel wall interaction, anemia, dialysis, and medications.Citation10 Renal failure had been proven to increase the risk of UGIB. Using the Taiwan National Health Insurance Research Database (NHIRD), Wu et al. found that patients with ESRD had a high long-term risk of developing peptic ulcer re-bleeding, especially in those taking ulcerogenic drugs or who also had H. pylori infection, ischemic heart disease, or liver cirrhosis.Citation11
However, the long-term risk of UGIB mortality in patients with uremia remained unclear. Therefore, this study aimed to investigate the association between mortality and UGIB in patients with uremia in order to realize the true incidence of mortality caused by UGIB and estimate the in-hospital mortality risk of UGIB in patients with uremia.
MATERIALS AND METHODS
Study Design
In this tertiary hospital-based retrospective cohort study, we reviewed the medical records of all patients with ESRD who were on maintenance hemodialysis and simultaneously had acute UGIB. We retrieved the admission diagnosis, discharge diagnosis, and laboratory data from multiple hospitalizations, including the emergency department and outpatient department from our hospital’s Clinical Informatics Research and Development Center between 31 December 2004 and 24 August 2010. These patients were followed until 31 December 2010. The study was approved by the institutional review board of Taichung Veterans General Hospital (No. CE11183-1). Informed consent was waived because there was no breach of privacy, and the study did not interfere with clinical decisions related to patient care.
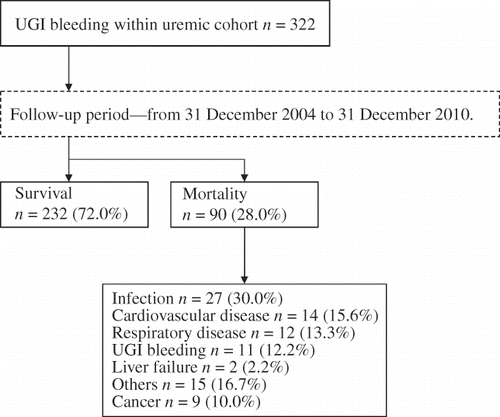
A total of 675 ESRD-related hospitalizations of patients who were on maintenance hemodialysis >3 months were documented for the 322 patients who were recruited at the Taichung Veterans General Hospital. The exclusion criteria were age <20 years, previous gastric resection or vagotomy, esophageal or gastric variceal bleeding, or gastric cancer within the first 2 years of the index hospitalization. The uremia cohort with UGIB was separated into survival (n = 232) and mortality (n = 90) groups according to the primary endpoint of this study, which was the all-cause mortality and risk estimation ().
Study Population and Subjects
All hospitalized patients with UGIB had one of the following diagnosis codes among their first three discharge diagnoses as defined by the International Classification of Diseases, 9th Revision (ICD-9): esophageal, gastric, duodenal, peptic, or gastrojejunal bleeds (esophageal, 530.4, 530.7, 530.82; hematemesis, 578.0; gastric ulcer, 531.00, 531.10, 531.20, 531.40, 531.50, 531.60, 531.80; duodenal ulcer, 532.00, 532.10, 532.20, 532.40, 532.50, 532.60, 532.80; peptic ulcer, 533.00, 533.10, 533.20, 533.40, 533.50, 533.60, 533.80; gastrojejunal ulcer, 534.00, 534.10, 534.20, 534.40, 534.50, 534.60, 534.80; gastritis/duodenitis with bleeding, 535.X1). Hypertension (HTN), cardiovascular (CV) disease, and numbers of chronic diseases within 19 evaluated chronic diseases were defined based on the admission and discharge diagnoses as well as a review of the patient’s medication such as oral antihypertensive agents, antiplatelets, or anticoagulants for their cardiovascular diseases. If patients were readmitted to our hospital or transferred to another hospital within 3 days due to UGIB, they were included in the same course. Patients with rehospitalization due to two or more UGIB episodes were assigned to the UGI re-bleeding group. In the patients with UGIB and ESRD who were on maintenance hemodialysis or those with a bleeding tendency, we usually used heparin-free hemodialysis. The procedure consisted of priming the artificial kidney with 2500 U of heparin in 500 cc of normal saline. The general type and dose of heparin during maintenance hemodialysis was an intravenous loading dose of 1000 U of heparin in 2 cc and a maintenance dose of 500 U of heparin in 1 cc for the entire course of hemodialysis.
Outcomes
Our primary endpoint was the all-cause mortality among patients with UGIB who were undergoing hemodialysis. We applied Cox proportional hazards model analysis to predict the outcome of UGIB in patients undergoing hemodialysis.
Data Analyses
All data analyses were performed using SPSS statistics 17.0 software (SPSS, Inc., Chicago, IL, USA). Chi-square test was used to compare demographic data between the subjects in the survival and mortality groups at the end of the follow-up study. The independent samples t test was used to analyze continuous variables. Cumulative incidence analysis was conducted using the Kaplan–Meier method. Cox proportional hazards model analysis was used to identify the predictive factors of outcomes in patients with uremia and UGIB. A p value <0.05 was considered significant.
RESULTS
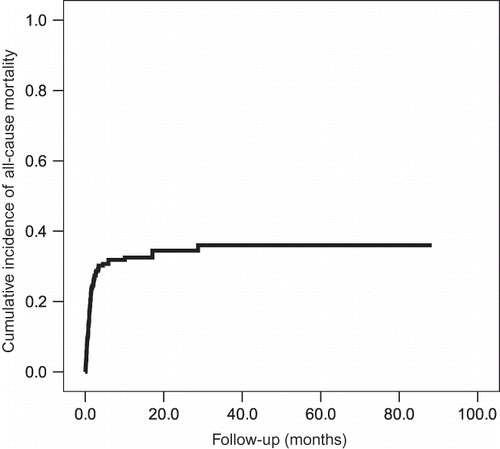
The survival and mortality rates of the patients with UGIB were 72.0% and 28.0%, respectively (). The main causes of mortality included infection (n = 27), CV disease (n = 14), respiratory disease (n = 12), UGIB (n = 11), liver failure (n = 2), others (n = 15), and cancer (n = 9). The all-cause mortality risk of the patients with UGIB who were undergoing hemodialysis was high: first-month mortality, 13.7%; sixth-month mortality, 26.7%; and first-year mortality, 27.0% ().
shows the baseline characteristics of the patients with UGIB in the uremia group. Patient age was older (73.0 ± 13.2 vs. 69.5 ± 14.3, p = 0.044) and hospital stay was longer (>14 days, 70.0% vs. 50.0%, p = 0.001) in the mortality group than in the survival group. The number of infection events during hospitalization was higher in the mortality group than in the survival group (65.6% vs. 35.3%; p < 0.001). The percentage of patients with single episodic UGIB in the mortality group was higher than that in the survival group (84.4% vs. 72.4%; p = 0.029). The percentage of patients with HTN and type 2 diabetes mellitus (DM), respectively, was higher in the UGIB survival group than in the mortality group (51.7% vs. 32.2%, p = 0.002; 17.2% vs. 5.6%, p = 0.011). The mean duration of follow-up in the UGIB mortality group was significantly shorter than that in the survival group (1.00 vs. 6.40 months, p < 0.001). However, there were no significant differences in any of the other variables between the UGIB survival and mortality groups.
Table 1. Baseline characteristics of patients with UGIB in the uremia group.
shows the laboratory data of patients with UGIB in the uremia group based on mortality and survival. The abnormal white blood cell (WBC) count was relatively higher in the mortality group than those in the survival group (56.7% vs. 36.2%, p = 0.001). A higher percentage of patients in the mortality group than in the survival group had a serum albumin level of <3 g/dL (81.1% vs. 50.4%, p < 0.001).
Table 2. Laboratory data of patients with UGIB in the uremia group based on survival and mortality.
There was no significant difference in the use of acid suppressive drugs (H2 receptor antagonists), except for proton-pump inhibitors, which was associated with a high percentage of mortality in the patients with UGIB (). There was also no significant difference in the use of ulcerogenic drugs associated with mortality in the patients with UGIB, except for NSAID use, which was associated with lower mortality (). Using the Cox regression models, we found that the high mortality of the UGIB group was significantly correlated with older age (adjusted HR = 1.02, 95% CI = 1.01–1.04), female sex (adjusted HR = 1.62, 95% CI = 1.05–2.51), infection during hospitalization (adjusted HR = 1.85, 95% CI = 1.13–3.03), single episodic UGIB (adjusted HR = 2.00, 95% CI = 1.08–3.70), abnormal WBC (adjusted HR = 1.59, 95% CI = 1.03–2.45), albumin level ≤ 3 g/dL (adjusted HR = 2.67, 95% CI = 1.51–4.72), and use of proton-pump inhibitors (adjusted HR = 2.41, 95% CI = 1.16–4.99) (). The use of NSAIDs was associated with low mortality (adjusted HR = 0.39, 95% CI = 0.19–0.81) ().
Table 3. Acid suppressive drugs and ulcerogenic drugs used by patients with uremia in the UGIB group based on survival and mortality.
Table 4. Cox proportional hazards model analysis for predicting UGIB outcomes in patients with uremia.
DISCUSSION
The incidence of acute nonvariceal UGIB among patients on dialysis is generally thought to be higher than that in the general population.Citation2,9 Here we conducted a retrospective cohort study to determine the risk factors for UGIB-related mortality among patients with ESRD. Although Haimanot reported that the incidence of UGIB among patients with ESRD is 21.3–24.0 per 1000 per year and accounted for 3–7% of all deaths irrespective of the study period,Citation7 our study showed a high mortality rate associated with UGIB among patients with ESRD (first-month mortality, 13.7%; sixth-month mortality, 26.7%; and first-year mortality, 27.0%). This result was similar to the finding in a study by Yang that revealed an overall 30-day mortality of 11.8%.Citation9 A multivariate analysis conducted by Cheung found that ESRD in addition to high-risk ulcer stigmata was an independent predictor of peptic ulcer re-bleeding.Citation6 Wu showed that ESRD with liver cirrhosis was associated with a higher risk of cumulative incidence of UGIB re-bleeding, which was not discussed in this study.Citation11 Less than 15% of patients underwent H. pylori examination. The inclusion of these patients might have confounded the results to some extent because adequate endoscopic diagnosis and therapy were not routinely performed during UGIB, and H. pylori-negative idiopathic bleeding tends to be related to the patient’s comorbidities.Citation5,6
Our findings were also comparable to the results of a study that investigated UGIB risk prediction in patients with ESRD and reported age and undernourishment (serum albumin per 1 mg/dL decrease) as risk factors.Citation7 Our data revealed that factors of high mortality in the patients with UGIB and uremia were older age, female sex, high infection rate during hospitalization, single episodic UGIB, more abnormal WBC count, and poor nutrition. However, multivariate analysis suggested that the use of proton-pump inhibitors worsened the outcome because of the potential for confounding the indication for treating the UGIB. NSAID use also had a lesser effect on all-cause mortality. In contrast to Wu’s findings, which showed that peptic ulcer re-bleeding significantly contributed to all-cause mortality in patients with ESRD who were undergoing hemodialysis compared with a matched cohort,Citation11 we found that single episodic UGIB carried the greatest risk of mortality. We postulate that the bleeding mechanism in patients with ESRD with several comorbidities may differ from that in the general population with comorbidities. The unpublished data show that patients in the UGIB re-bleeding group with infection during hospitalization and serum alkalosis appeared to be at a greater risk of death, which was compatible with the U-curve relationship finding of the Dialysis Outcomes and Practice Patterns Study (DOPPS)Citation12 in which a greater midweek predialysis serum bicarbonate level was associated with an increased risk of mortality and hospitalization.Citation12 Furthermore, an inverse correlation had been demonstrated, whereby the normalized protein catabolic rate (nPCR) was greater in patients on dialysis who were more acidotic.Citation13 The bleeding diathesis of patients with uremia is a significant clinical concern, especially when surgery and other invasive procedures are required.Citation14 Several factors may contribute to uremic bleeding, namely complex platelet dysfunction with abnormal platelet–vessel wall interaction, abnormal production of nitric oxide, uremic toxins, anemia, and drug treatment.Citation10,14
Although endoscopic findings were available in all of our retrospective reviews, they were difficult to categorize. The findings in the esophagus included short mucosa breaks, small-caliber white varices in the lower third without red coloring, esophageal erosion, esophagitis, and linearly dilated veins over the lower third of the esophagus. The gastric findings included shallow gastric ulcers, superficial gastritis with micro-ulcers, hyperemic fundus of the stomach, pangastritis, and angiodysplasia. Duodenitis and duodenal ulcers were also mentioned. The main causes of mortality and the mean duration from UGIB to death included infection (2.79 months), CV disease (1.09 months), UGIB (4.04 months), respiratory system (1.08 months), and cancer (1.43 months).
Finally, the aim of this study was to identify the factors associated with the high mortality rate in patients with UGIB undergoing hemodialysis. One of the strengths of this study was the use of a hospital-based cohort design in a well-defined population that allowed for the identification of mortality risk and long-term monitoring. Thus, in patients with UGIB, older age, female sex, infection during hospitalization, single episodic UGIB, abnormal WBC count, and poor nutritional status were found to significantly affect mortality. The increased morbidity and mortality may be associated with the high incidence of a preexisting comorbid condition.
This longitudinal study had several limitations. First, we identified patients with UGIB based on ICD-9 coding, which might have resulted in some misclassification. However, such misclassification was probably nondifferential and would likely to have resulted in less statistical power in detecting the estimated effects. Second, we could not definitively ascertain whether these patients experienced an episode of UGIB before or after the index episode. Third, not all patients had the endoscopic findings that were used to make a definite diagnosis, including active bleeding, visible vessels, and adherent clots. Fourth, it was quite possible that the lower mortality rates in the re-bleeding group were a result of the sicker patients dying during the first bleed. As such, survival bias existed.
CONCLUSION
In conclusion, patients with ESRD admitted with primary UGIB had a profoundly increased risk of all-cause in-hospital mortality during the follow-up period. Older age, female sex, infection during hospitalization, single episodic UGIB, abnormal WBC count, and poor nutritional status increased the all-cause mortality in patients with UGIB.
ACKNOWLEDGMENTS
The authors are grateful to the Clinical Informatics Research and Development Center and the Biostatistics Task Force of Taichung Veterans General Hospital, Taichung, Taiwan, R.O.C., for its assistance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The authors thank the Taichung Veterans General Hospital, Taiwan, R.O.C., for providing the grants (TCVGH-1003606A and TCVGH-1013602A) for this study.
REFERENCES
- Kurata JH, Nogawa AN. Meta-analysis of risk factors for peptic ulcer. Nonsteroidal anti-inflammatory drugs, Helicobacter pylori, and smoking. J Clin Gastroenterol. 1997;24:2–17.
- Puneet S, Gagan K, Rahul N, . Chronic kidney disease and end-stage renal disease predict higher risk of mortality in patients with primary upper gastrointestinal bleeding. Am J Nephrol. 2012;35:216–224.
- Toke ABG. GI bleeding risk in patients undergoing dialysis. Gastrointest Endosc. 2010;71:50–52.
- Lassen A, Hallas J, Schaffalitzky de Muckadell OB. Complicated and uncomplicated peptic ulcers in a Danish county 1993–2002: a population-based cohort study. Am J Gastroenterol. 2006;101:945–953.
- Wong GL, Wong VW, Chan Y, . High incidence of mortality and recurrent bleeding in patients with Helicobacter pylori-negative idiopathic bleeding ulcers. Gastroenterology. 2009;137:525–531.
- Cheung J, Yu A, LaBossiere J, Zhu Q, Fedorak RN. Peptic ulcer bleeding outcomes adversely affected by end-stage renal disease. Gastrointest Endosc. 2010;71:44–49.
- Wasse H, Gillen DL, Ball AM, . Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney Int. 2003;64:1455–1461.
- Chan FK, Leung WK. Peptic-ulcer disease. Lancet. 2002;360:933–941.
- Yang JY, Lee TC, Montez-Rath ME, . Trends in acute nonvariceal upper gastrointestinal bleeding in dialysis patients. J Am Soc Nephrol. 2012;23:495–506.
- Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19:317–322.
- Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ, Lin JT. Long-term peptic ulcer re-bleeding risk estimation in patients undergoing hemodialysis: a 10-year nationwide cohort study. Gut. 2011;60:1038–1042.
- Bommer J, Locatelli F, Satayathum S, . Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis. 2004;44:661–671.
- Galbusera M, Remuzzi G, Boccardo P. Treatment of bleeding in dialysis patients. Semin Dial. 2009;22:279–286.
- Chauveau P, Fouque D, Combe C, . Acidosis and nutritional status in hemodialyzed patients. French Study Group for Nutrition in Dialysis. Semin Dial. 2000;13:241–246.