Abstract
Background: Identifying the risk factors is important in prevention of urinary tract infections (UTIs) in children. The aim of this study is to evaluate the association of UTI and idiopathic hypercalciuria (IHC). Methods: Two hundred and twenty-four children aged between 1 month and 16 years and diagnosed to have UTI were evaluated for urinary calcium excretion. The children were diagnosed to have IHC if their urinary calcium/creatinine ratios in at least two different spot urine samples were >0.6 between 0–1 year old and ≥0.21 over 1 year or daily calcium excretion >4 mg/kg. Results: The frequency of IHC was found to be 16.7%. Family history of urolithiasis, parental consanguinity, presentation with abdominal pain, loss of appetite, and discomfort were found to be significantly higher in the IHC group. No association was found between IHC and the recurrence of UTI, presence of vesicoureteral reflux, renal scar formation, and the prognosis. Conclusions: IHC should be considered among the risk factors for UTI and should be investigated particularly in patients with family history of urinary stones and suggestive complaints of IHC.
INTRODUCTION
Urinary tract infection (UTI) is one of the most common bacterial infections in children which are detected at least once in 8% of girls and 2% of boys until the age of 7.Citation1 Both the short- and long-term complications of UTI are important. Long-term complications are usually associated with their occurrence at younger age and recurrence. They include renal damage, decreased glomerular filtration rate, delay in renal development, hypertension, proteinuria, pregnancy complications, and chronic renal failure.Citation2 Besides the early diagnosis and treatment, prevention of infection is also required for the protection of renal functions. Therefore, it is of great importance to consider the predisposing factors and the associated risk factors for UTI.
Idiopathic hypercalciuria (IHC) is one of the most common causes of urolithiasis in children with a frequency of 3–6% in normal population. It is defined as the urinary calcium excretion of more than 4 mg/kg in 24 h or calcium/creatinine ratio >0.21 over 1 year of age in normocalcemic children.Citation3 Although the children with IHC are generally asymptomatic, they usually present with complaints such as macroscopic or microscopic hematuria, recurrent UTI, voiding dysfunction, urine incontinence, recurrent abdominal pain, suprapubic tenderness, and lumbar pain in addition to urolithiasis.Citation4 The damage in the urinary epithelium caused by the microcrystals was thought to be responsible for these urinary symptoms. However, the relationship between UTI and IHC has been rarely reported in the literature.Citation5–7 In this study, we aimed to determine the prevalence of IHC among the children diagnosed to have UTI and to investigate the association of IHC with age, gender, family history, and symptoms.
PATIENTS AND METHODS
The children who were diagnosed to have UTI at least one time and free from infection for the last 8 weeks were included in the study. The study was approved by the Ethics Committee of Dr. Sami Ulus Maternity and Children’s Health and Diseases Training and Research Hospital and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
The demographic data, clinical and laboratory findings, and imaging studies of the patients were investigated. Past and recent medical histories, current medications, family histories of urolithiasis, and parental consanguinity were recorded. The patients with chronic diseases that were bedridden for a long time and that were on diuretic or corticosteroid-like medications were not included in the study. After the informed consents were obtained from the parents, spot urine samples were collected from all children. Calcium and creatinine concentrations were measured, and urinary calcium/creatinine ratios (UCa/UCr) were calculated in all samples. The values of UCa/UCr higher than 0.6 between 0–1 year old and 0.21 over the age of 1 year were suspected to show hypercalciuria (HC) and 24-h urine samples were collected from these children. Those with urinary calcium excretion more than 4 mg/kg/day were diagnosed to have HC. When it was difficult to collect 24-h urine samples from the infants and small children, spot urine UCa/UCr measurements were repeated and those with at least two high values were diagnosed to have HC.
In patients with HC, complete urinalysis was performed and blood urea nitrogen, creatinine, uric acid, sodium, chloride, potassium, calcium, phosphorus, alkaline phosphatase, and magnesium levels were determined together with the levels of thyroid-stimulating hormone, total thyroxine, parathyroid hormone, 25-hydroxy vitamin D3, and calcitonin levels and blood gasses in order to identify the predisposing factors for HC. Urinary excretions of sodium, potassium, creatinine, phosphate, magnesium, oxalate, citrate, and amino acids were also analyzed in either 24-h or spot urine samples according to the age of the patient. The patients who were detected to have secondary HC were excluded from the study.
The mean UCa/UCr values of the patients and their age and sex distributions were investigated and the frequency of IHC among patients with UTI was determined. The distribution of symptoms, family histories, presence of predisposing urinary system abnormalities and their progression during the follow-up period, and the recurrence of UTI’s (2 or more UTI) were compared between the HC and normocalciuric (NC) patients.
Statistical analyses were performed using Statistical Package for the Social Sciences for Windows version 16.0 (SPSS Inc., Chicago, IL, USA). Variables were presented as mean ± standard deviation, number (n), or percentage (%). Kolmogorov–Smirnov test was used to verify the normal distribution of the quantitative variables. Student’s t-test or one-way analysis of variance (ANOVA) test was used for normally distributed variables, whereas Mann–Whitney U-test or Kruskal–Wallis test was used for non-normally distributed variables. Chi-square test, Student’s t-test, Mann–Whitney U-test or Pearson correlation analysis was used to test for the statistical significance. A p-value < 0.05 was considered statistically significant.
RESULTS
There were 224 children (58 males, 166 females) included in the study. They were between 1 month and 16 years of age. The mean age of them was 6.41 ± 3.90 years, and it was significantly lower in boys (3.96 + 2.97) than in girls (7.27 + 3.83) (p < 0.01). The mean follow-up period of the patients was 33.83 ± 24.77 months.
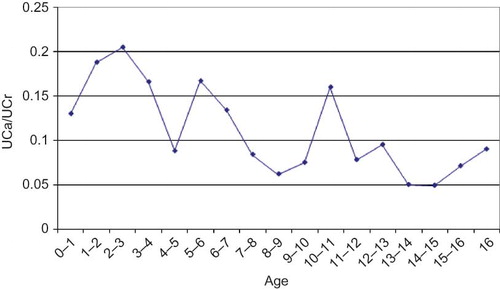
The mean UCa/UCr ratio of the patients was found to be 0.12 ± 0.17. It was significantly higher in boys (0.17 ± 0.24) than in girls (0.11 ± 0.13) (p = 0.025). It was found that the highest values (0.20 ± 0.20) were detected in 2–3 years of age, whereas the lowest values (0.049 ± 0.046) were detected in 14–15 years (). There was a weak negative but statistically significant correlation between the age and UCa/UCr ratios with Pearson correlation analysis (r = −0.225, p = 0.001). Thirty-six patients were diagnosed to have IHC. The frequency of IHC among the patients with UTI was found to be 16.7%. The mean UCa/UCr ratio was 0.39 ± 0.26 in patients with IHC while it was 0.07 ± 0.07 in NC group.
The distribution of the symptoms and findings during UTI among groups with IHC and NC are presented in . The most common symptom in the IHC group was abdominal pain followed by fever, dysuria, loss of appetite, vomiting, and foul-smelling urine. The frequencies of abdominal pain, loss of appetite, and foul-smelling urine were significantly higher in the IHC group as compared to the NC group. The mean UCa/UCr ratios were significantly higher in patients with abdominal pain, loss of appetite, and discomfort than those without these complaints.
Table 1. The distribution of the clinical findings according to the HC and NC groups and urinary calcium/creatinine ratios according to the presence of these findings.
As shown in , parental consanguinity and family history of urolithiasis were found to be significantly higher in the IHC group than the NC group. However, there were no significant differences between the IHC and the NC groups in terms of predisposing renal abnormalities detected by renal ultrasonography, voiding cystourethrography (VCUG), and renal scan with dimercaptosuccinic acid (DMSA). There were also no significant differences in mean UCa/UCr ratios and mean daily urinary calcium excretions between the patients with and without pathological renal findings with ultrasonography, DMSA, and VCUG ().
Table 2. Comparison of the family histories and urinary system abnormalities between the groups with HC and NC.
Table 3. Comparison of the groups with and without pathological findings with the imaging methods in terms of urinary calcium/creatinine ratios and daily urinary calcium excretion.
Table 4. The features of the children with single and recurrent urinary tract infection.
Control DMSA scans were recorded in 60 children with initial pathological findings in the follow-up period and no significant differences could be found between the IHC and NC groups in terms of the progression of DMSA findings (p = 0.978).
Of the ten children with IHC and VUR, nine had grade 1 to 3 reflux, and one had grade 4 to 5 reflux. Of the 46 NC children with VUR, 30 had grade 1 to 3 reflux, whereas 16 of them had grade 4 to 5 reflux. There was no statistically significant difference between the groups for the degree of VUR (p = 0.112). Control VCUG findings were recorded for 45 children with VUR. There was also no significant difference in VUR progression between the IHC and the NC groups (p = 0.476).
The features of the subjects with single UTI and recurrent UTI are presented in . The number of girls with recurrent UTI was significantly higher than the boys. The mean UCa/UCr ratio of those who had single UTI was significantly higher than that of those with recurrent UTI. However, there was no difference when the age factor was included (p > 0.05).
DISCUSSION
IHC is mostly associated with various clinical findings other than urolithiasis in childhood. It is reported to have an important role as an underlying factor in patients presented with hematuria, recurrent UTI, dysuria, abdominal and flank pain, voiding dysfunction, and enuresis. Moreover, renal calyceal microlithiasis or nephrolithiasis develops in some of these patients during the long-term follow-up.Citation8 In recent studies, remarkable improvement has been observed in the majority of these clinical conditions with the treatment of IHC.Citation8–16 Since many of these conditions including UTI may also lead to significant morbidity and mortality in children, identification of IHC is of great importance.
The underlying mechanism leading to UTI in IHC includes the impairment of uroepithelium with calcium oxalate microcrystals. Thus, the function of uroepithelium, which has certain roles in the host defense such as bactericidal activity, integrity of the inflammatory response, and local IgA secretion, diminishes. In order to initiate the antibacterial response, close contact is required between the bacteria and epithelial cell surface. Calcium oxalate monohydrate crystals slow down the defense mechanism by blocking this contact and form safe barrier that prevents the mechanical removal of bacteria via urine flow.Citation5–7 In a previous study, it was observed that treatment of IHC with thiazide diuretics prevented recurrence of UTI in six girls with IHC and recurrent UTI.Citation5 In another study conducted on 124 children with IHC, Vachvanichsanong et al.Citation10 observed UTI in 40% (n = 50) of the children and 78% (n = 39) of them had recurrent UTI. After treatment of IHC, no recurrence of UTI was observed in 83% of 29 patients in a follow-up period of 6 years.
There are a limited number of studies investigating the frequency of IHC in children with UTI.Citation5–7,10,17–19 In a study from Serbia, Stojanovic et al.Citation6 detected the rate of IHC as 21% in children with UTI. In a study from Turkey conducted by Biyikli et al.Citation17 on 75 children with recurrent UTI over the age of 5 years, IHC was reported in 32 (43%) patients. Sadeghi-Bojd et al.Citation19 found the frequency of IHC as 30% in Iranian children with UTI which was significantly higher than the control subjects. In our study, IHC was detected in 36 (16%) of 224 children with UTI which seems to be lower than the other studies including the study from Turkey. This difference may be due to the increased number of patients in our study which was almost three times than the other studies and the inclusion of all age groups. In any case, our result is also higher than the prevalence of IHC in Turkey which was reported to be between 5.8% and 9.6%.Citation20,21 These results suggest that IHC should be investigated in children with UTI.
The amount of urinary calcium excretion in childhood varies not only from country to country, but also between the different regions of the same country. This can be attributed to the difference between the children in terms of race, geographic area, and particularly eating habits.Citation22–27 In a study performed by So et al.Citation28 with 368 children, the mean UCa/UCr ratio was found as 0.10 in children aged between 19 months and 6 years, and 0.09 in children aged ≥7 years. In another study conducted by Moore et al.,Citation29 the mean UCa/UCr ratio was found as 0.06 ± 0.062 where the majority of the subjects were black children. Vachvanichsanong et al.Citation30 found that the mean UCa/UCr ratio was 0.10 in Thai children aged between 5 and 10 years and 0.06 between 10 and 15 years. In the present study, the mean UCa/UCr ratio (0.12 ± 0.17) was slightly higher than the values of the above mentioned. This may be attributed to the fact that our study population consisted of the children with UTI and it may further support the association of UTI and IHC.
In addition to the environmental and dietetic factors, inheritance has an important role in the development of IHC. It has been shown that familial IHC has an autosomal dominant inheritance and the frequency of IHC and urolithiasis in the other family members and first degree relatives of these patients are higher. The prevalence of urolithiasis in the families of these patients has been reported between 34% and 59%.Citation17,18,31,32 In our study, there was a significant difference between the IHC and NC groups in terms of the rate of family history of urolithiasis. Furthermore, the rate of parental consanguinity was found to be higher in the IHC group. Thus, the children with UTI should be evaluated for urinary calcium excretion when they have family histories of urolithiasis and parental consanguinity.
The effect of age and gender on urinary calcium excretion and IHC has been evaluated in several studies with conflicting results. There are some studies showing that UCa/UCr ratio decrease with age while there are also studies reporting that there is no association between these parameters.Citation23–26,29 Similarly, while many studies show no relationship between the gender and UCa/UCr ratio, there are also different studies demonstrating that IHC is more common in girls in some of them, and in boys in some others.Citation20,22,24,29,33,34 In our study, there was a weak negative but statistically significant correlation between the urinary calcium excretion and age. However, no significant difference between the boys and girls was found in the prevalence of IHC although the mean UCa/UCr ratios were significantly higher in boys.
In this study, we also investigated the complaints of the patients during UTI and their associations with UCa/UCr ratios. The mean UCa/UCr ratios were found to be significantly higher in patients with abdominal pain, loss of appetite, and discomfort as compared to those without these complaints. Recurrent abdominal pain is considered as an important symptom of IHC. Vachvanichsanong et al.Citation32 reported the recurrent abdominal and flank pain in 42% of the patients with IHC and these complaints were decreased or either completely resolved in 86% of them after treatment. The frequency of abdominal and flank pain in children with IHC was reported as 19% by Esfahani et al.,Citation35 60.3% by Tabel et al.Citation11 and 40.3% by Selimoglu et al.Citation20 In the present study, the prevalence of abdominal pain was found to be 36.2% in the NC group and 61.1% in the IHC group. Hematuria, which can also occur in the absence of urolithiasis, is also an important clinical symptom in the subjects with IHC and has been reported to be 21%–50.3%.Citation8,10,11,32 Stojanovic et al.Citation6 reported that, 6.7% of the 75 subjects with UTI had microscopic hematuria, and all of them had IHC. In a study performed by Lopez et al.,Citation18 the frequency of hematuria was reported to be 12% in patients with recurrent UTI and IHC. In another study conducted by Biyikli et al.,Citation17 hematuria was reported as 35% in children with IHC and recurrent UTI, whereas this ratio was 33% in those without IHC. In our study, the frequency of hematuria was reported to be 16.7% in patients with IHC, and there was no significant difference with NC group. The frequency of IHC was also 20.7% in children who presented with hematuria. Thus, when etiological factors are investigated, IHC should be considered particularly in the patients who present with abdominal pain, loss of appetite, discomfort, and hematuria.
In the present study, no significant differences could be found between the IHC and the NC groups in terms of predisposing renal abnormalities, degree of vesicoureteral reflux (VUR), and progression of renal scars and VUR during the follow-up period. Furthermore, no significant differences were obtained in urinary calcium excretions between the patients with and without VUR. In a study performed on the children with recurrent UTI, although there was no significant difference, urinary system abnormalities were detected in 37.5% of patients with IHC and 19% of those without IHC.Citation17 The difference in these results may be due to the selection of study populations and age groups. Since our study included all age groups of childhood, predisposing urinary abnormalities were also frequent in NC group. In another study performed on 46 children with VUR but not UTI, the frequency of IHC was found higher than the normal population; however, there was no association between the degree of VUR and the scar formation with IHC like our study.Citation7
Stojanovic et al.Citation6 found that the frequency of IHC in patients with recurrent UTI was significantly higher (44%) than the children who had a single UTI (10%). Vachvanichsanong et al.Citation10 observed UTI in 40% of the children with IHC and 78% of them had recurrent UTI. In a study from Turkey, performed by Tabel et al.Citation11 on 131 children with IHC, 23% of them had UTI. In our study, 66.7% of patients with IHC had recurrent UTI. However, there was no difference between the patients who had single UTI and recurrent UTI, when the age factor was included. This result should not exclude the necessity of investigating the IHC particularly in children with recurrent UTI, because our follow-up period is not consistent enough for the very young children included in our study.
In conclusion, the frequency of IHC is higher in patients with UTI than its prevalence in normal populations. In the light of this and other studies, it can be suggested that the recurrence of UTI may be reduced by the treatment of IHC in patients with UTI which may lead to serious and high-cost complications such as renal failure. Thus, the children with UTI should be evaluated for urinary calcium excretion particularly when they have a family history of urinary stones and who present with the complaints of abdominal pain, loss of appetite, discomfort, and hematuria.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Williams G, Craig JC. Prevention of recurrent urinary tract infection in children. Curr Opin Infect Dis. 2009;22:72–76.
- Jones KV. Urinary tract infection in infancy and childhood. In: Davison AM, Cameron JS, Grünfeld JP, Kerr DNS, Ritz E, Winerals CG, eds. Oxford Textbook of Clinical Nephrology. 2nd ed., New York: Oxford University Press; 1998:1261–1275.
- Srivastava T, Schwaderer A. Diagnosis and management of hypercalciuria in children. Curr Opin Pediatr. 2009;21:214–219.
- Lau KK. Clinical manifestations of pediatric idiopathic hypercalciuria. Front Biosci (Elite Ed). 2009;1:52–59.
- Heiliczer JD, Canonigo BB, Bishof NA, Moore ES. Noncalculi urinary tract disorders secondary to idiopathic hypercalciuria in children. Pediatr Clin North Am. 1987;34(711):718.
- Stojanovic VD, Milosevic BO, Djapic MB, Bubalo JD. Idiopathic hypercalciuria associated with urinary tract infection in children. Pediatr Nephrol. 2007;22:1291–1295.
- Garcia-Nieto V, Siverio B, Monge M, Toledo C, Molini N. Urinary calcium excretion in children with vesicoureteral reflux. Nephrol Dial Transplant. 2003;18:507–511.
- Penido MG, Diniz JS, Moreira ML, . Idiopathic hypercalciuria: presentation of 471 cases. J Pediatr (Rio J). 2001;77:101–104.
- Polito C, Iolascon G, Nappi B, Andreoli S, La Manna A. Growth and bone mineral density in long-lasting idiopathic hypercalciuria. Pediatr Nephrol. 2003;18:545–547.
- Vachvanichsanong P, Malagon M, Moore ES. Urinary tract infection in children associated with idiopathic hypercalciuria. Scand J Urol Nephrol. 2001;35:112–126.
- Tabel Y, Mir S. The long-term outcomes of idiopathic hypercalciuria in children. J Pediatr Urol. 2006;2:453–458.
- Sikora P, Glatz S, Beck BB, . Urinary NAG in children with urolithiasis, nephrocalcinosis, or risk of urolithiasis. Pediatr Nephrol. 2003;18:996–999.
- Heilberg IP, Schor N. Renal stone disease: causes, evaluation and medical treatment. Arq Bras Endocrinol Metabol. 2006;50:823–831.
- Garcia-Nieto V, Navarro JF, Monge M, Garcia-Rodriguez VE. Bone mineral density in girls and their mothers with idiopathic hypercalciuria. Nephron Clin Pract. 2003;94:89–93.
- Penido MG, Lima EM, Marino VS, Tupinamba AL, França A, Souto MF. Bone alterations in children with idiopathic hypercalciuria at the time of diagnosis. Pediatr Nephrol. 2003;18:133–139.
- Vachvanichsanong P, Malagon M, Moore ES. Urinary incontinence due to idiopathic hypercalciuria in children. J Urol. 1994;152:1226–1228.
- Biyikli NK, Alpay H, Guran T. Hypercalciuria and recurrent urinary tract infections: incidence and symptoms in children over 5 years of age. Pediatr Nephrol. 2005;20:1435–1438.
- Lopez MM, Castillo LA, Chavez JB, Ramones C. Hypercalciuria and recurrent urinary tract infection in Venezuelan children. Pediatr Nephrol. 1999;13:433–437.
- Sadeghi-Bojd S, Hashemi M. Hypercalciuria and recurrent urinary tract infections among children in Zahedan, Iran. J Pak Med Assoc. 2008;58(11):624–626.
- Selimoglu MA, Alp H, Bitlisli H, Orbak Z, Energin M, Karakelleoglu C. Urinary calcium excretion of children living in the east region of Turkey. Turk J Pediatr. 1998;40:399–404.
- Sonmez F, Akcanal B, Altincik A, Yenisey C. Urinary calcium excretion in healthy Turkish children. Int Urol Nephrol. 2007;39:917–922.
- Kruse K, Kracht U, Kruse U. Reference values for urinary calcium excretion and screening for hypercalciuria in children and adolescents. Eur J Pediatr. 1984;143:25–31.
- Ghazali S, Barratt TM. Urinary excretion of calcium and magnesium in children. Arch Dis Child. 1974;49:97–101.
- Stapleton FB, Noe HN, Roy S, Jerkins G. Hypercalciuria in children with urolithiasis. Am J Dis Child. 1982;136:675–678.
- Sargent JD, Stukel TA, Kresel J, Klein RZ. Normal values for random urinary calcium to creatinine ratios in infancy. J Pediatr. 1993;123:393–397.
- Esbjörner E, Jones IL. Urinary calcium excretion in Swedish children. Acta Paediatr. 1995;84:156–159.
- Siegel SR, Hadeed A. Renal handling of calcium in the early newborn period. Kidney Int. 1987;31:1181–1185.
- So NP, Osorio AV, Simon SD, Alon US. Normal urinary calcium/creatinine ratios in African-American and Caucasian children. Pediatr Nephrol. 2001;16:133–139.
- Moore ES, Coe FL, McMann BJ, Favus MJ. Idiopathic hypercalciuria in children: prevalence and metabolic characteristics. J Pediatr. 1978;92:906–910.
- Vachvanichsanong P, Lebel L, Moore ES. Urinary calcium excretion in healthy Thai children. Pediatr Nephrol. 2000;14:847–850.
- Ozkaya O, Buyan N, Erol I, . The relationship between urinary calcium, sodium, and potassium excretion in full-term healthy newborns. Turk J Pediatr. 2005;47:39–45.
- Vachvanichsanong P, Malagon M, Moore ES. Recurrent abdominal and flank pain in children with idiopathic hypercalciuria. Acta Paediatr. 2001;90:643–648.
- Manz F, Kehrt R, Lausen B, Merkel A. Urinary calcium excretion in healthy children and adolescents. Pediatr Nephrol. 1999;13:894–899.
- Rath B, Aggarwal MK, Mishra TK, Talukdar B, Murthy NS, Kabi BC. Urinary calcium creatinine ratio and hypercalciuria. Indian Pediatr. 1994;31:311–316.
- Esfahani ST, Madani A, Siadati AA, Nabavi M. Prevalence and symptoms of idiopathic hypercalciuria in primary school children of Tehran. Iran J Pediatr. 2007;17:353–358.