Abstract
Objective: C-reactive protein (CRP) has been implicated as a possible mediator of the association between periodontitis and several systemic diseases. This study evaluated the impact of nonsurgical periodontal treatment on the serum levels of CRP in chronic kidney disease (CKD) patients on hemodialysis. Methods: A total of 77 CKD patients on hemodialysis were included in this study. At baseline, periodontal examination was assessed for all the patients, and chronic periodontitis was defined through clinical attachment level and probing pocket depth, according to the American Association of Periodontology. Nonsurgical periodontal treatment was performed and serum levels of CRP were evaluated at baseline and 8 weeks after periodontal treatment. Results: Periodontal treatment resulted in significant reductions in CRP levels (p < 0.001). The difference between pre- and posttreatment CRP concentrations did not show any significant relationship with the severity of periodontitis. Conclusions: Periodontitis is an important source of systemic inflammation in CKD patients. Nonsurgical periodontal treatment can effectively reduce the serum level of CRP in these patients.
INTRODUCTION
Periodontitis is a chronic inflammatory disease of infectious origin contributing to the destruction of the supporting structures of the teeth, including the periodontal ligament, as its consequences.Citation1 It includes local inflammation and is associated with systemic inflammatory response.Citation2 Ultimately, it may lead to tooth loss.Citation1 In this infectious disease, Gram-negative bacteria are recognized as the main pathogens.Citation3 Periodontal diseases are estimated to affect more than 70% of the general population.Citation4 Different researches have shown that about 10% of the adult population and about 30% of the persons over the age of 50 years suffer from severe periodontitis.Citation5
Chronic kidney disease (CKD) is a major public health problem that its rising prevalence and consequences have made it a worldwide challenge. Studies revealed that approximately 25 million people in the United States may have CKD.Citation6 Its prevalence is on the increase in the industrialized countries.Citation7
CKD is characterized by a progressive and irreversible failure in renal function which is associated with a reduced glomerular filtration rate.Citation8 It has high morbidity and mortality. For example, the mortality rate of chronic dialysis patients in the United States is reported to be 24% per year.Citation9 The increasing rate of morbidity and mortality is mostly because of atherosclerotic complications, especially among younger patients. The conditions that contribute to these complications include myocardial infarction, cardiac arrest, and cardiac arrhythmia that may lead to sudden death and cerebrovascular accidents.Citation7 The treatments of CKD include diet modification, recognition and treatment of systemic complications, dialysis, and finally, kidney transplantation.Citation2,Citation8
Nowadays renal replacement therapies have improved the survival rate of end-stage renal disease (ESRD) patients, so the number of the patients seeking renal replacement therapy as well as dental treatments has increased. Both chronic renal disease and renal replacement therapy that includes hemodialysis, peritoneal dialysis, or renal transplantation can affect periodontal tissue.Citation7 That is, these patients usually show increased levels of plaque, calculus, and gingival inflammation. Also, gingival hyperplasia and possible increased prevalence and severity of destructive periodontal diseases are reported in ESRD patients.Citation5 A positive correlation between the periodontal index and the age and time of dialysis has been obtained in different studies.Citation10
Several studies have been conducted, assessing the presence of dental plaque, calculus, gingival bleeding, and probing pocket depth (PD) in these patients.Citation11 Also, dental management of renal patients is greatly affected by their chronic disease and renal replacement therapies.Citation7
Furthermore, CKD and periodontitis can have significant reciprocal effects.Citation7 This means that chronic periodontitis by its overall systemic inflammatory effects can influence the management of CKD patients on hemodialysis maintenance therapy.Citation7 It is not yet fully understood whether periodontitis plays a role as a risk factor for CKD. However, in many cases, the common etiology of kidney diseases such as diabetes mellitus, hypertension, pyelonephritis, glomerulonephritis, nephrosclerosis, polycystic kidney disease, and collagen vascular diseases cannot explain the onset of CKD. So other unknown etiologic factors may be suspected.Citation12
The production of cytokines, prostaglandins and, in some cases, acute-phase reagents play an important role in the pathogenesis of periodontitis.Citation13 This means that in spite of the chronic nature of periodontitis, acute-phase elements also take part in its immunophathogenesis.Citation14 This explains that how periodontitis as an unrecognized infection can sometimes induce an acute-phase response, enhance a systemic inflammatory process, and elevate levels of acute inflammatory mediators.Citation14 By this way systemic levels of inflammatory mediators maybe affected by persistent localized infections such as periodontitis.Citation14 Among the acute-phase elements in inflammatory process, C-reactive protein (CRP) is one of the most interested mediators.Citation15
CRP is one of the nonspecific mediators that can be produced in various inflammatory situations. Its concentration increases rapidly following inflammation and raised serum levels may be detected within 6 h. The potential stimuli that provoke its production include trauma, (unknown) infection and/or inflammation, smoking, obesity, and hypoxia.Citation14 Due to its various provoking situations, CRP can be used as a reliable biomarker for clinical purposes in asymptomatic individuals.Citation16 It is primarily synthesized in the liver.Citation17 Hepatic CRP synthesis is upregulated by proinflammatory cytokines that are released locally at the site of infection or inflammation.Citation18 Some studies have shown that human gingiva is also able to produce CRP in situ.Citation17
CRP is useful in making decisions regarding diagnosis, monitoring, and treatment of inflammatory processes and accompanying diseases.Citation14 Numerous studies have suggested that CRP maybe a more powerful indicator for cardiovascular diseases (CVDs) than traditional risk factors such as low-density lipoprotein (LDL) in the general population as well as in the CKD patients.Citation19 Many studies have shown a strong relationship between CRP levels and cardiovascular problems; i.e., CRP is considered as a key marker of atherosclerosis, and elevated levels of CRP (e.g., >2.1 mg/L) is a risk factor for CVDs.Citation20 This association has led to the hypothesis that controlling of inflammation and thus reducing the CRP levels may be beneficial in attenuating atherothrombotic process.Citation20 The mechanism of CRP involvement in the pathogenesis of CVDs is not obvious; however, CRP may activate the complement system and participate in atheroma formation.Citation21
Due to the fact that periodontitis is associated with CVDs and several studies have shown the association between periodontitis and the risk of atherosclerosis, myocardial infarction and strok,Citation22 there is also interest in studying the relationship between periodontitis and CRP. A positive association between chronic periodontal infections and elevated CRP levels has been established in numerous studies.Citation23,Citation24
Based on different studies, it is proposed that elevated levels of CRP in periodontitis can partly explain the association between periodontitis and CVDs.Citation14
There is sustained interest in the field of periodontology that if progression and treatment of periodontitis can affect serious systemic diseases.Citation25 Despite the fact that poor periodontal health has been linked to higher systemic CRP levels, at present, the question that whether successful periodontal treatment can reduce CRP levels remains to be answered. It is expected that due to significant rise in CRP levels in the presence of periodontal disease, the potential for reduction with treatment is greater in periodontal patients.Citation4
Upon the hypothesis that part of the inflammatory response seen in the CKD patients originates from the chronic periodontitis and act through an increase in the expression of inflammatory markers such as CRP, the researchers of this study were motivated to design this study. Therefore, the purpose of this study was to investigate the impact of initial periodontal treatment on the serum levels of CRP in patients with chronic periodontitis and CKD and also to determine whether there is a causal association between chronic periodontitis activity and high serum levels of CRP. Recently, a high-sensitivity assay (<3–5 mg/L CRP concentrations) has been developed to detect CRP below what was previously considered the normal range.Citation4,Citation26 This recent development has made the detection of even mild elevations of CRP possible.Citation27 As this high-sensitivity test was applied in this study, the results of this study are expected to be comparable to that of other studies.
MATERIALS AND METHODS
The present study was an interventional, uncontrolled clinical trial. The study population included 77 CKD patients, on hemodialysis, who were recruited from the hemodialysis centers of Shahid Faghihi, Namazi and Haj Ebrahimi hospitals in Shiraz. All the subjects included in the study were informed about the aim of the study, risks, and benefits and signed an informed consent form. The Ethics Committee of Shiraz University of Medical Sciences reviewed and approved this study. Also, all the medical and dental procedures were in accordance with the ethical standards for human experimentation established by the Declaration of Helsinki.Citation28 The inclusion criteria for the volunteers to take part in the study consisted of diagnosed as ESRD patients according to clinical practice guidelines in the National Kidney Foundation, being on regular maintenance hemodialysis (since 6 months ago) and at least two times per week and the presence of at least 15 teeth. The exclusion criteria for the study were known systemic diseases that interfere with kidney replacement therapies (such as HIV-positivity, lupus erythematosus, rheumatoid arthritis, and diabetes), the history and/or the presence of other infections, treatment with any medication known to affect serum levels of CRP, pregnancy or lactation in females, the need for antibiotic prophylaxis, systemic antibiotic prescription, or any periodontal treatment in the preceding 6 months and smoking.
At the baseline visit, a questionnaire including the following information was completed: age, sex, occupation, complete medical history, medications used, and dialysis status. All the volunteers received a full-mouth periodontal examination, except for the third molars, performed at six sites per teeth (mesiobuccal, buccal, distobuccal, mesiolingual, lingual, and distolingual) by one trained examiner. The periodontal examinations included the following parameters: the number of teeth, probing PD, gingival recession, and clinical attachment level (CAL). These examinations were conducted by Williams probe and the measurements were approximated to the nearest millimeter.
Also, plaque index (PI) for assessing supra gingival plaque and bleeding index (BI) for detecting the marginal gingival bleeding were performed for all teeth.
According to the American Academy of Periodontology classificationCitation29 and considering CAL, participants were classified as gingivitis, mild, moderate, and severe periodontitis. Also generalized chronic periodontitis was diagnosed by the presence of >30% periodontal involvement and localized clinical periodontitis by the involvement of <30% of the sites. Gingivitis was defined as no presence of CAL, no site with PD ≥ 5 mm and apparent clinical signs of gingival inflammation.
After baseline examinations, all the subjects received conservative nonsurgical periodontal treatment. This phase was initiated with oral hygiene instructions including proper brushing and flossing and introducing other interproximal cleansing aids, when needed, and continued as scaling and root planning with ultrasound devices and manual instruments (Gracey curettes), polishing, and subgingival curettage. The treatment procedure was performed by a periodontist in 1-h sessions and repeated according to the need of each patient. Local anesthesia was used when necessary. All other necessary dental treatments including extraction of hopeless teeth and restoration of carious lesions were carried out in order to decrease the accumulation of microbial plaque. Upon completing the periodontal treatment, based on the need of the patients, a follow-up program was carried out, for the additional oral hygiene instructions and supragingival prophylaxis.
Nonfasting blood samples were collected from the participants at two periods: at the baseline visit before periodontal treatment and 8 weeks after initial treatment. The samples were immediately stored at –80˚C and processed blindly by staff. The serum CRP concentration (high sensitivity) was quantified in milligram per liter by nephelometric method, using MININEPHTM human CRP kit (The Binding Site Ltd., Birmingham, UK). The test was performed according to the instructions of the manufacturer. The approximate measuring range was 3.5–112 mg/L at a sample dilution of 1/40. The sensitivity limit was 0.44 mg/L when using a 1/5 sample dilution.
Statistical Analyses
Paired-sample t-test was used for comparing the means of CRP levels before and after the treatment. For detecting the relation between pre- and posttreatment CRP levels difference and sex of the patients, Mann–Whitney test was used. Also, Kruskal–Wallis test was used for detecting this relation between pre- and posttreatment difference of CRP levels and the severity of periodontitis. The relation between the severity of periodontitis and pretreatment levels of CRP was also determined by Kruskal–Wallis test. The strength of linear relationship between pretreatment CRP levels and age, PI, and BI was assessed by Pearson’s correlation coefficient. Data were expressed as frequencies and means (±SD). P < 0.05 were considered statistically significant. All statistical analyses were carried out with IBM spss19.0 for windows software (SPSS Inc., Chicago, IL., USA).
RESULTS
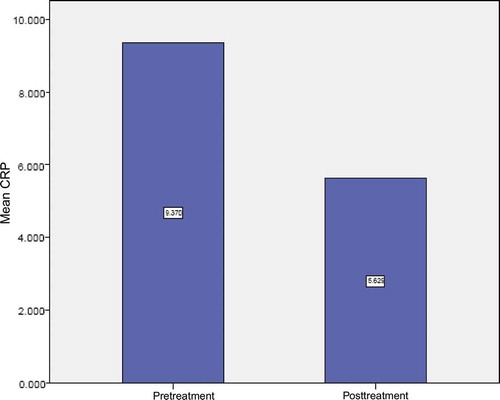
A total of 77 stable maintenance hemodialysis patients consisting of 52 (67.5%) males and 25 (32.5%) females with mean age of 44.35 ± 17.2 years (range 14–88 years) were included in this study. Periodontal examination revealed that gingivitis was present in 15.6%, mild periodontitis in 7.8%, moderate periodontitis in 46.8%, and severe periodontitis in 29.9% of the cases (). The mean CAL of mild periodontitis group was in the range of 1–2 mm, that of moderate periodontitis was in the range of 3–4 mm, and that of severe periodontitis was ≥ 5 mm. Mean PI and BI were 72.36 ± 20.98 and 72.09 ± 21.76, respectively. At baseline, the mean of CRP was 9.369 ± 11.25 mg/L, but its concentration did not show any significant relation neither to the severity of the periodontitis (p = 0.823) () nor to the BI ( p = 0.899). Eight weeks after periodontal treatment, the mean CRP concentration was 5.628 ± 4.79 mg/L. Comparison of CRP data before and after treatment showed significant reduction ( p < 0.001) ( and ). However, no significant relation was found between this difference and the severity of periodontitis ().
Table 1. Distribution of different periodontal problems (N = 77).
Table 2. Relation of pretreatment CRP levels and periodontal problem.
Table 3. Comparison of CRP levels before and after periodontal therapy.
Table 4. Relation of difference of pre- and posttreatment CRP levels and periodontal problem.
DISCUSSION
Over the last 50 years, dentists and physicians used to believe that periodontal infections were localized only to the marginal periodontium and that there was rarely systemic adverse effects.Citation30 But nowadays, the concept that progression and treatment of periodontitis can affect systemic diseases is the focus of sustained interest in the field of periodontology.Citation12
CKD patients suffer from undesired systemic outcomes such as cardiovascular problems that are associated with systemic inflammation.Citation31 Nevertheless, the origin and nature of this inflammation are not always identifiable.Citation32 There are many potential sources of inflammation in CKD patients who are on maintenance hemodialysis treatment. These include vascular access infection, urinary tract infection, dialysis water contamination, bio-incompatible membrane, sinusitis, otitis, and periodontal disease.Citation33
Local tissue destruction that occurs in periodontitis favors the systemic dissemination of periodontal pathogens and their products (e.g., lipopolysaccharides). This leads to the local production of inflammatory mediators, such as CRP, IL-6, IL-1, TNF-α, and PGE-2.Citation34 CRP represents as an accurate and reliable marker of the acute-phase response to infectious burdens and/or inflammation and it is also effectively used for the early detection of individuals at risk for future cardiovascular accidents.Citation14,Citation35 It has been shown that the acute-phase inflammatory response initiated by periodontitis can be measured by the serum level of CRP.Citation36
Although multiple mechanisms can explain elevated values of CRP in the ESRD patients, many of them experience high levels of CRP in the absence of apparent infection or inflammation.Citation18
Considering that periodontitis is a risk factor for atherosclerotic changes in CKD patients, the researchers have found it an important preventive measure to make an early diagnosis of periodontitis followed by periodontal treatment in the treatment course of CKD patients.Citation37
There are different studies that reveal the association between high serum levels of CRP and the severity of periodontitis in ESRD who are on hemodialysis.Citation7,Citation38,Citation39
Although there are numerous studies that have examined the effect of different periodontal treatment modalities on serum CRP levels in chronic periodontitis patients, this is scarcely performed on patients suffering from both periodontitis and CKD.
From the first category, many treatment studies show an effect on CRP levels in favor of periodontal treatment. Teixeira de Freitas et al.Citation40 have concluded from a meta-analysis that nonsurgical periodontal treatment can exert a reductive effect on the serum levels of CRP. These authors propose that the time of measuring CRP and the type and the extent of periodontal treatment can influence the results of the studies dramatically. Also, D’Aiuto et al.,Citation30,Citation35 Paraskevas et al.Citation36 reported that initial periodontal treatment can lead to a reduction in serum CRP levels.
On the contrary, some other studies failed to show this positive result. For example, in a meta-analysis performed by Loannidou et al.,Citation4 the information from randomized clinical trials and small single-cohort studies did not support the hypothesis that periodontal treatment can reduce systemic CRP levels. These reviewers concluded that the mean pretreatment level of CRP is an important determinant in the evaluation of research results. This means that not very high pretreatment levels of CRP, which was shown in most of the investigated studies (mean 2.6 mg/L), were not conductive to a large improvement after treatment.Citation25 This relatively low CRP level before treatment is in agreement with some other reports in the literature for chronic periodontitis patients with no known history of systemic diseases such as cardiovascular or cerebrovascular accidents. It is noteworthy to mention that some of the studies have shown that periodontal disease significantly affects CRP levels only in the patients with an apparent history of thromboembolic accidents.Citation41
Also, Loannidou et al.Citation4 have concluded that whether complete or inadequate periodontal treatment is performed for the patients has a detrimental effect on the systemic levels of CRP. This means that those patients with moderate and severe periodontitis who need more advanced periodontal treatment may not benefit much from nonsurgical periodontal treatment.Citation25
Yamazaki et al.Citation42 failed to demonstrate the positive effect of initial periodontal treatment on serum levels of CRP in Japanese population, although there was a tendency for CRP to decrease following successful periodontal treatment. These authors related their obtained results to the relatively small number of patients. The findings of their study were consistent with those of Idle et al.Citation43
Siribamrungwong et al.Citation39 found significant decrease in the serum levels of CRP 8 weeks after the completion of periodontal treatment in 30 maintenance hemodialysis patients. Vilela et al.Citation37 also reported the reduction of CRP, IL-6, and prohepcidin levels in both CKD and control patients 12 weeks following periodontal treatment. The reduction of inflammatory burden, as well as serum levels of CRP, was shown in the study. This indicates that periodontal treatment may constitute an important intervention therapy during the therapeutic course of CKD patients.Citation37
The present study showed that initial periodontal treatment was associated with a significant decrease in serum levels of CRP in CKD patients on hemodialysis. This finding was in agreement with the results of some other publications.Citation37,Citation39 During the study, patients were under observation and no important changes in the lifestyle, habits, medical health, or medications were noticed. So it can be proposed that different degrees of periodontal inflammation that were present in these patients and controlled by treatment, have contributed to their inflammatory burden.
According to the Centers for Disease Control and Prevention (CDC) recommendations, serum CRP levels less than 1 mg/L are considered low risk, 1 to 3 mg/L average risk and more than 3 mg/L high risk for the development of coronary heart disease.Citation20 On the other hand, some other investigators have proposed that CRP values less than10 mg/mL, in the general population, can be considered as normal. However, the more accepted CRP values are that, the range of 5–10 mg/L is 420 regarded as “high normal”. In fact, this range can be predictive of atherosclerotic complications with underlying inflammatory process.Citation44
The participants of this study had mean baseline CRP concentrations about 9.369± 11.25 that was in the range of high risk for developing CVDs. Although periodontal treatment significantly reduced the mean levels of CRP, it was still in the high risk limit after treatment (5.628 ± 4.79 mg/L). It can be proposed that given the needs of each patient, maybe more definitive periodontal treatment, could lower the serum level of CRP more efficiently. Recent studies have proposed that even a modest increase in CRP levels, such as those seen in periodontal patients, may predict a risky situation for atherosclerosis and CVDs.Citation45–48 In the present study the patients who present situations favoring the production of CRP were excluded from the study design. So the authors speculated that the relatively high pretreatment level of CRP that yields itself to the periodontal treatment, could be related to the presence of periodontal infection. Also, it is proposed that if this increased level of acute-phase response, left unchanged, in part may contribute to a higher risk for CVDs.
Also, based on the periodontal examination and classification, none of the subjects participated in this study were periodontally normal. The majority of the patients (84.4%) had periodontitis and the minority had different degrees of gingivitis (15.6%). This means that there were different levels of local periodontal inflammation in all subjects that may have contributed to the relatively mean high level of CRP in this population in comparison with other studies.
Regarding the prevalence of destructive periodontal diseases in the general population, and since periodontal examinations are not normally performed as a part of the routine medical assessment of ESRD patients on hemodialysis therapy, periodontitis can be introduced as an overlooked source of inflammation in these patients. The authors of this article would like to alert the physicians to the possible role of destructive periodontal diseases in the management of CKD and ESRD patients. So preventive measures such as regular visits to a dental professional and improvement of oral hygiene procedures as well as definitive periodontal treatment modalities and maintenance of patients in a health situation, after treatment, are highly recommended to be considered as a part of medical treatment of CKD patients.
In conclusion, chronic destructive periodontitis is an important source of systemic inflammation in the maintenance of hemodialysis patients. It can induce an acute-phase response, including elevation in CRP values, which in turn is associated with atherosclerotic complications. Effective management of periodontitis can improve systemic inflammation in this population and may lower the risk of associated systemic complications. The results of this investigation can be used in the design and implementation of other longitudinal follow-up studies with a larger sample size, in order to evaluate the effect of periodontal therapy on the CRP serum levels as well as on the other systemic inflammatory markers and also on the kidney performance criteria. In studies with extended samples, the effect of severity of periodontal destruction on baseline CRP levels and on its changes after treatment could be better evaluated.
ACKNOWLEDGMENTS
The authors thank Dr. Mehrdad Vossoughi from the Dental Research Development Center for the statistical analyses and Dr. Ehya Amal saleh for improving the use of English in the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The authors thank the vice-chanceller of Shiraz University of Medical Sciences for supporting the research (Grant#88-5104). The article is based on the thesis by Dr. Noozhan Karimi.
REFERENCES
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820.
- D’Aiuto F, Ready D, Tonetti MS. Periodontal disease and C-reactive protein-associated cardiovascular risk. J Periodontal Res. 2004;39:236–241. doi:10.1111/j.1600-0765.2004.00731.x
- Kshirsagar AV, Kl M, Elter JR, Beck JD, Offenbacher S, Falk RJ. Periodontal disease is associated with renal insufficiency in the atherosclerosis risk in communities (ARIC). Am J Kidney Dis. 2005;45(4):650–657.
- Ioannidou E, Malekzadeh T, Dongari-Bagtzoglou A. Effect of periodontal treatment on serum C-reactive protein levels: A systematic review and meta-analysis. J Periodontol. 2006;77(10):1635–1642.
- Gjermo P. 1998. Epidemiology of periodontal diseases in Europe. J Parodontol d’Implantol Orale. 17:111–121.
- D’Aiuto F, Parkar M, Nibali L, Suvan J, Lessem J, Tonetti MS. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: results from a randomized controlled clinical trial. Am Heart J. 2006;151:977–984.
- Craig RG. Special Review in Periodontal Medicine Interactions between chronic renal disease and periodontal disease. Oral Dis. 2008;14:1–7.
- Crawford PW, Lerma EV. Treatment options for end stage renal disease. Prim Care. 2008;35:407–432.
- US. Renal Data System: USRDS. Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; 2006.
- Kshirsagar AV, Craig RG, Beck JD, Severe periodontitis is associated with low serum albumin among patients on maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2007;2(2):239–244 [Epub January 24, 2007].
- Bayraktar G, Kurtulus I, Kazancioglu R, et al. Evaluation of periodontal parameters in patients undergoing peritoneal dialysis or hemodialysis. Oral Dis. 2008;14:185–189.
- Litlle JW, Falace DA, Miller CS, Rhodus NL. Dental Management of the Medically Compromised Patient. 7th ed. St. Louis, MO: Mosby; 2008:180 p.
- Linden GJ, McClean K, Young I, Evans A, Kee F. Persistently raised C-reactive protein levels are associated with advanced periodontal disease. J Clin Periodontol. 2008;35:741–747.
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812.
- Blake GJ, Ridker PM. 2002. Inflammatory bio-markers and cardiovascular risk prediction. J Int Med. 252:283–294.
- RidkerP M, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-dentistry lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565.
- Lu Q, Jin L. 2010. Human gingiva is another site of C-reactive protein formation. J Clin Periodontol. 37:789–796.
- Craig RG, Spittle MA, Levin NW. Importance of periodontal disease in the kidney patient. Blood Purif. 2002;20:113–119.
- Stenvinkel P, Chung SH, Heimburger O, Lindholm B. Malnutrition, inflammation, and atherosclerosis in peritoneal dialysis patients. Perit Dial Int. 2001;21(Suppl 3):157–162.
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14): 1387–1397.
- Torzewski J, Torzewski M, Bowyer DE, et al. C-reactive protein frequency colocalizes with the terminal complement complex in the intima of early atherosclerotic lesions of human coronary arteries. Arterioscler Thromb Vasc Biol. 1998;18:1383–1392.
- Söder PO, Söder B, Nowak J, Jogestrand T. 2005. Early carotid atherosclerosis in subjects with periodontal diseases. Stroke. 36:1195–1200.
- Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72(9):1221–1227.
- Wu T, Trevisan M, Genco RJ, Dorn JP, Falkner KL, Sempos CT. Periodontal disease and risk of cerebrovascular disease: The first national health and nutrition examination survey and its follow-up study. Arch Intern Med. 2000;160(18):2749–2755.
- Hilana Paula Carillo Artese. Celso Oliveira de Sousa. Ronir Raggio Luiz. Carmelo Sansone. Maria Cynésia Medeiros de Barros Torres. Braz Oral Res. 2010;24(4):449–454.
- Rogowski O, Vered Y, Shapira I, Hirsh M, Zakut V, Berliner S. Introducing the wide range C-reactive protein (wr-CRP) into clinical use for the detection of microinflammation. Clin Chim Acta. 2005;358(1–2):151–158.
- Helal I, Zerelli L, Krid M, Comparison of C-reactive protein and high-sensitivity C-reactive protein levels in patients on hemodialysis. Saudi J Kidney Dis Transpl. 2012;23(3): 477–483.
- World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. J Am Med Assoc. 1997;277(11):925–926.
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Bon Perim/onto. 1999;4:1.
- D’Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005;84(3):269–273.
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi:10.1161/hc0902.104353.
- Bl P, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi:10.1016/S0140-6736(05)67728-8
- Bradbury BD, Critchlow CW, Weir MR, Stewart R, Krishnan M, Hakim RH. Impact of elevated C-reactive protein levels on erythropoiesis-stimulating agent (ESA) dose and responsiveness in hemodialysis patients. Nephrol Dial Transplant. 2009;24(3):919–925 [Epub October 7, 2008].
- Kshirsagar AV, Offenbacher S, Moss KL, Barros SP, Beck JD. Antibodies to periodontal organisms are associated with decreased kidney function: the dental atherosclerosis risk in communities study. Blood Purif. 2007;25:125–132. doi:10.1159/000096411
- D’Aiuto F, Parkar M, Andreou G, Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83(2):156–160.
- Paraskevas S, Huizinga JD, Loss BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35:277–279. doi:10.1111/j.1600-051X.2007.01173.x
- Vilela EM, Bastos JA, Fernandes N, Ferreira AP, Chaoubah A, Bastos MG. Treatment of chronic periodontitis decreases serum prohepcidin levels in patients with chronic kidney disease. Clinics (Sao Paulo). 2011;66(4):657–662.
- Chen LP, Chiang CK, Chan CP, Hung KY, Huang CS. Does periodontitis reflect inflammation and malnutrition status in hemodialysis patients? Am J Kidney Dis. 2006;47(5):815–822.
- Siribamrungwong M, Puangpanngam K. Treatment of periodontal diseases reduces chronic systemic inflammation in maintenance hemodialysis patients. Ren Fail. 2012;34(2):171–175 [Epub January 9, 2012].
- Freitas CO, Gomes-Filho IS, Naves RC, Influence of periodontal therapy on C-reactive protein level: a systematic review and meta-analysis. J Appl Oral Sci. 2012;20(1):1–8.
- Glurich I, Grossi S, Albini B, et al. Systemic inflammation in cardiovascular and periodontal disease: comparative study. Clin Diagn Lab Immunol. 2002;9:425–432.
- Yamazaki K, Honda T, Oda T, Effect of periodontal treatment on the C-reactive protein and proinflammatory cytokine levels in Japanese periodontitis patients. J Periodontal Res. 2005;40(1):53–58.
- Ide M, McPartlin D, Coward PY, Crook M, Lumb P, Wilson RF. Effect of treatment of chronic periodontitis on levels of serum markers of acute-phase inflammatory and vascular responses. J Clin Periodontol. 2003;30:334–340.
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454.
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97(5):425–428.
- Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97(20):2007–2011.
- Danesh J, Muir J, Wong YK, Ward M, Gallimore JR, Pepys MB. Risk factors for coronary heart disease in acute phase proteins: a populations-based study. Eur Heart J. 1999;20:954–959.
- Lagrand WK, Visser CA, Hermens WT, C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999;100(1):96–102.