Abstract
Background: hRenalase may degrade catecholamines and regulate sympathetic tone and blood pressure (BP). The aim of the study was to assess dopamine (DA), norepinephrine (NE), and renalase in 75 hemodialysis (HD) and 26 peritoneal dialysis (PD) patients and their correlations with heart rate (HR), BP, a type of hypotensive therapy, and residual renal function. Methods: Renalase, DA, NE were studied using commercially available assays. Results: Renalase and NE were higher and DA was lower in dialyzed groups comparing to healthy volunteers. Hemodialysis patients had lower NE and higher renalase level. Norepinephrine was higher in anuric patients in HD group. Renalase correlated with dialysis vintage and inversely with residual diuresis. Dopamine correlated with residual diuresis in the whole study cohort, with HR in PD patients, with renalase in HD patients. Norepinephrine correlated with aortic diameter in PD patients. Norepinephrine was significantly higher in patients with coronary artery disease (CAD) in HD group. Hemodialysis population with CAD had lower NE and higher DA and renalase level than their PD counterparts. In the follow up, 27% of HD group died. Cardiac death was diagnosed in 17% and there was higher renalase level than in noncardiac death. Conclusions: Elevated level of circulating renalase in dialysis patients is rather related to kidney function and the sympathetic nervous system hyperactivity found in this population. The real excess of renalase in the pathogenesis of cardiovascular disorders in patients with chronic kidney disease still remains to be proven. If confirmed, it may give a new way for pathophysiological therapy.
INTRODUCTION
Chronic kidney disease (CKD) is associated with a high risk of cardiovascular disease (CVD).Citation1 It is the result of the presence of many risk factors.Citation2 One of them is the sympathetic nervous system (SNS) overactivity which correlates with increased vascular resistance and systemic blood pressure (BP).Citation3 Plasma norepinephrine (NE) levels are predictive of both survival and incidents of CVD in end-stage renal disease. The elevated level of catecholamines in CKD is the result of not only overspill, but also of reduced catecholamines clearance. Norepinephrine clearance is reduced by 20% in mild renal failure and by up to 40% in hemodialysis (HD) patients. Correction of uremia by successful kidney transplantation does not normalize sympathetic nerve activity.Citation4 Elevated SNS activity contributes to the CVDs development also in whole population. In renal patients, despite of the influence on CVD, it also aggravates renal failure progression, what results in the poor prognosis.Citation5
In 2005, the group of Xu and DesirCitation6 discovered and described a new protein, released by kidney and named it—renalase. They observed in vitro study degradation of catecholamines by renalase and they predicted that it may have a significant hemodynamic effect in vivo. Renalase infusion in rats caused a dose-dependent decrease in cardiac contractility, HR, and BP and prevented a compensatory increase in peripheral vascular tone. Using the Western blot test, the group of Xu and DesirCitation6 showed qualitatively a lower serum renalase in patients with CKD maintained HD comparing to healthy individuals. The recent, from 2012, study of group of DesirCitation7 in 5/6 nephrectomized rats, using in vitro enzymatic assays and in vivo administration of recombinant RNLS showed that it acts as NADH-dependent oxidase that lowers BP by degrading plasma epinephrine. They also found that renalase 1 had low activity against dopamine (DA) and did not metabolize NE. On the other hand, the study made on patients after renal transplantation found that serum renalase level was significantly higher in kidney transplant recipients than in healthy volunteers.Citation8 The genetic studies confirmed the connection between renalase and hypertension.Citation9,Citation10
Taking into account available data about the relationship between renalase, catecholamines, and BP, we decided to assess, for the very first time, in vivo study together plasma catecholamines—DA and NE concentration and serum renalase level in HD group and peritoneal dialysis (PD group) patients and to evaluate correlations of those parameters with BP control, heart rate (HR), a type of hypotensive therapy, and residual renal function.
MATERIALS AND METHODS
We included in the study 101 patients from the Dialysis Center in Bialystok, Poland: 75 HD group in the mean age of 64.4 years (45.3% males) and 26 PD group patients in the mean age of 54.6 years (53.8% males).
All patients were informed about the aim of the study and gave their informed consent. The study was approved by the Medical University Ethic Committee. BP and HR was measured in HD group—before and after HD session and in PD group—during routine ambulatory control, in the sitting position using automatic manometer. The arithmetic average of three measurements taken in the different days was used for the analysis. Then the mean arterial pressure (MAP) was calculated using the formula: MAP = [(2×diastolic) + systolic]/3. The well controlled BP was assessed according to K/DOQI guideline as lower than 140/90 mmHg in PD patients and in HD patients before HD session and lower than 130/80 mmHg after that.Citation11 Body mass index (BMI) was calculated according to the dry weight. The residual diuresis was assessed based on the 24-hour urine collection. The presence of residual renal function was defined as the urine collection above 100 mL/24 h. The kind of hypotensive drugs was collected from the individual prescription cards. The blood for the estimation of the renalase and catecholamines concentration and parameters of blood morphology, electrolytes, and lipids levels was taken once, in PD group—while routine ambulatory visit and in HD group—before the HD session in the middle of three dialysis sessions (when BP, HR and, weight were also assessed). The enzyme-linked immunosorbent assay (ELISA) kit made by Uscn Life Science Inc. China, using a monoclonal antibody specific to renalase was taken to assess serum renalase level. Catecholamines were estimated in plasma. Blood samples were withdrawn from the upper limb vein, using Vacutainer system tubes with EDTA. The references ranges for plasma catecholamines are: NE < 600 pg/mL, DA <100 pg/mL according to the manufacturer of diagnostic assays used for the purpose of this study (Noradrenaline ELISA kit, Dopamine ELISA kit—from Labor Diagnostica Nord GmbH & Co. KG, Germany). To obtain the normal ranges for renalase and catecholamine in these ELISA assays 27 healthy volunteers were studied. Laboratory tests were performed using standard methods in the central hospital laboratory. Statistica 9.0 Poland software was used for statistical analysis, the Shapiro-Wilk test was used to determine the normal distribution. T-Student test, U Mann-Whitney test, and ChiCitation2 test were used for comparison of the two groups. The presence of correlations was estimated using Spearman or Pearson coefficient, as appropriate.
RESULTS
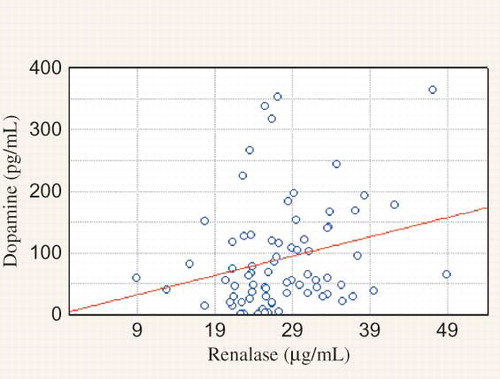
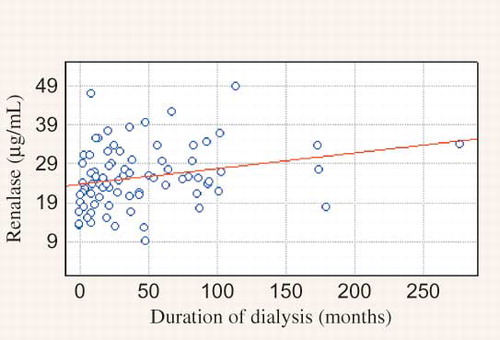
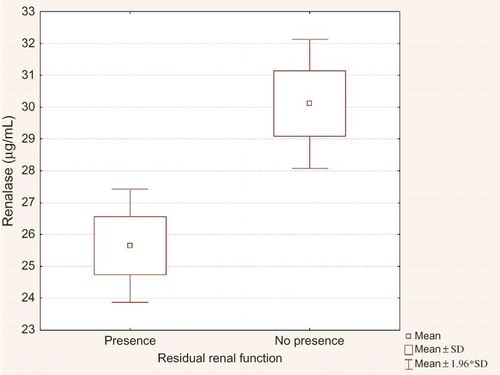
Clinical and biochemical data of the studied population are presented in the . We observed significantly higher MAP in PD when compared to HD group and higher HR in HD than in PD group. HD patients were older. There were no significant differences in sex, the presence of residual renal function and percentage of patients with abnormal BP control (>140/90 mmHg) between studied groups. The main hypotensive drugs were β-blockers and calcium-channel blockers. Dialysis patients had lower DA concentration and higher NE and renalase level than healthy volunteers (). There was no difference between HD and PD group in DA level. NE was lower in HD group. Renalase was higher in HD comparing to PD group (). There were correlations between DA and HR, calcium, HDL-cholesterol, and LDL-cholesterol level in PD patients, in HD group—between DA and renalase level () and in the whole study cohort—between DA and diuresis (). The NE correlated with aortic diameter in PD patients (r = −0.657, p = 0.003). In HD group NE was higher in anuric patients [0.64 (0.2–2.1) vs. 0.43 (0.1–1.4) ng/mL, p = 0.019]. Renalase correlated with the duration of dialysis therapy () and inversely with the presence of residual renal function (). No correlation between DA, NE, renalase and, BP was found. Taking into consideration the presence of coronary artery disease (CAD), we found the significant higher NE level in patients with the presence of CAD comparing to those without in HD group [Me = 0.66 (0.3;2.1) ng/mL vs. Me = 0.47 (0.1; 1.4) ng/mL, p = 0.02]. The HD patients with CAD had higher level of DA [Me = 54.79 (16.9;363.3) pg/mL vs. Me = 36.18 (0.8; 79.2) pg/mL, p = 0.0415] and renalase—29.02 ± 6.8 μg/mL vs. 17.23 ± 4.7 μg/mL, p = 0.0001) and lower NE level [Me = 0.66 (0.3;2.1) ng/mL vs. Me = 1.12 (0.6; 3.9) ng/mL, p = 0.0246] than PD patients with CAD. Until now, 27% of HD group died (n = 20). Cardiac death was diagnosed in 17% (n = 13). There was higher renalase level in those with cardiac death (28.47 ± 4.4 μg/mL vs. 24.41 ± 2.8 μg/mL, p = 0.0214) in HD group. Because of too few deaths (n = 3) we did not analyze the PD group.
Figure 2. Correlation between residual renal function and dopamine (DA) concentration (r = −0.2035, p = 0.04227).
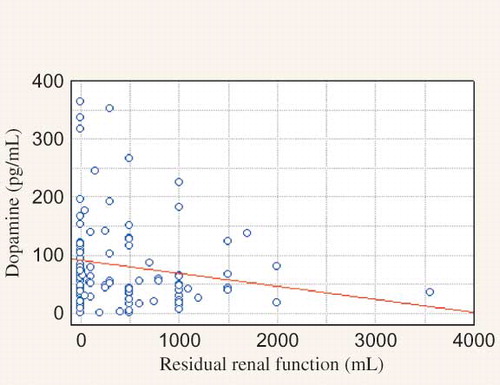
Table 1. Main characteristic of the study cohort.
Table 2. Renalase, norepinephrine (NE), dopamine (DA) concentration in study cohort and control group.
Table 3. Renalase, norepinephrine (NE), and dopamine (DA) concentration in the study groups.
DISCUSSION
Hypertension is associated with enhanced SNS activity as reflected by elevated plasma NE, muscle sympathetic nerve activity and whole-body NE spillover. Massuo et al.Citation12 reported that NE was significantly higher in those who had a strong family history of hypertension comparing to subjects without it. It was also found in longitudinal studies of 10 years that plasma NE level is predictive of future BP elevation.Citation13
Sympathetic nervous system activity is also raised in patients with CKD and correlates with increased BP.Citation14 It appears to be driven by diseased kidney while nephrectomy or renal denervation corrected BP and sympathetic nerve activity. What is interesting is that the recent study of Domingos and EscaldaCitation15 revealed autonomic dysfunction even in recurrent kidney stone formers what contributed with higher HR and BP comparing to healthy controls. One of the important determinants of SNS hyperactivity is abnormal renal sodium excretion and activation of the renin-angiotensin-aldosterone system and then volume overloud, which per se may also play a role. The activation of chemoreceptors within the kidney by uremic metabolites also plays a role in SNS overactivity.Citation16 The elevated level of catecholamines in CKD is the result of not only overspill in the mechanisms that involve inhibition of nitric oxide followed by increased angiotensin II and increased in sympathetic afferent outflow from diseased kidneys, but also of reduced catecholamine clearance.Citation3,Citation16 Norepinephrine clearance is reduced by 20% in mild renal failure and by up to 40% in HD patients. In our cohort of dialysis, patients plasma NE concentration was significantly higher comparing to the healthy volunteers and, what is interesting is that, in HD group it was significantly higher in anuric patients. Zoccali et al.Citation3 also found frankly elevated plasma NE level in a substantial proportion of dialysis patients and in their study it predicted survival and incidents of CVD. SNS activation and vagal withdrawal may be linked to rhythm disturbances and lead to a sudden cardiac death, a frequent cause of mortality in patients with CKD. Norepinephrine affects cardiomyocytes and could be responsible for left ventricular hyperthrophy.Citation16 In our study, we found a significant correlation between NE and aortic diameter in PD patients. We also indicated that MAP was significantly higher in PD group together with the rise of NE level when compared to HD patients. There was also higher NE level in PD patients with CAD than HD patients with CAD.
Dopamine is a well characterized neurotransmitter and plays an important role as a regulator of salt and water excretion by the kidney.Citation17 Its action is mediated by a family of five transmembrane G protein-coupled receptors. They are expressed in the mammalian kidney. Dopamine serves as a counterregulatory factor to angiotensin II in the kidney. It inhibits renal renin expression and angiotensin II mediated proximal tubule reabsorption and AT1 receptor expression.Citation18 The intrarenal dopaminergic system is responsible for regulating over 50% of net renal salt and water excretion when salt take is increased. The dysfunction in DA production and receptor function is associated with human essential hypertension and a number forms of animal genetic HTN.Citation19 We found lower DA level in whole dialyzed population relative to healthy volunteers. Moreover, we indicated the significant relationship between DA concentration and residual renal function. We also found higher DA level in HD patients with CAD comparing to PD group with CAD.
In 2005, Xu et al.Citation6 discovered a new, released by kidney, protein—renalase. The specific signal of renalase was detected in renal glomeruli, proximal tubules, and cardiomyocytes as well as in other tissue, i.e., endothelium, adipose tissue, etc.Citation20 At that time they postulate that renalase represents a new class of FAD-containing monoamine oxidase. In in vitro study, they observed the renalase degraded catecholamines and they predicted that it may have a hemodynamic effect in vivo. Renalase infusion in rats caused a dose-dependent decrease in cardiac contractility, HR, and BP and prevented a compensatory increase in peripheral vascular tone. Experimental data showed that renalase KO mouse were hypertensive, had tachycardia and higher catecholamines levels than wild-type animals and were prone to myocardial damage during acute ischemia.Citation21 However, their findings were questioned.Citation22 Model of heart failure in rats provide us with information of increased renal renalase expression together with a rise in NE levels.Citation23 However, authors did not study serum creatinine. In the recent study, from 2012, the group of Desir,Citation7 using in vitro enzymatic assays and in vivo—subcutaneous administration of recombinant renalase to 5/6 nephrectomized rats, found that hRenalase 1 preferentially metabolized epinephrine, using NADH as a cofactor, which had a strong hypotensive effect—similar to that of 5 mg/kg enalapril. What is interesting is that, no effect on HR was observed. Dopamine was metabolized in an extremely low rate and, surprisingly, there was no measurable activity of hRenalase 1 against NE—the strong CVD risk factor. The authors did not observe the same effect on BP after the subcutaneous administration of recombinant renalase to spontaneously hypertensive stroke-prone (SHRSP) rats. In turn, Pandini et al.,Citation24 using two different methods, were unable to prove monoamine oxidase activity of RNLS. In addition, very recently Baroni et al.Citation25 showed that renalase belonged to the p-hydroxybenzoate hydroxylase structural family of flavoenzymes, contained non-covalently bound FAD with redox features suggestive of a dehydrogenase activity, and was not a catecholamine-degrading enzyme, either through oxidase or NAD(P)H-dependent monooxygenase reactions.
Xu et al.,Citation6 using the Western blot test, showed qualitatively a lower serum renalase in 8 HD patients comparing to 4 healthy individuals. Wang et al.Citation26 also showed a diminished renalase expression in one patient with CKD, one HD patient, and 2 healthy controls. On the other hand, the recent study found that serum renalase level was significantly higher in kidney transplant recipients than in healthy volunteers.Citation27 The predictors of serum renalase was kidney function, age, time after transplantation, and diastolic BP. Przybylowski et al.Citation28 showed similar findings in heart transplant recipients. In their study, predictor of renalase was only kidney function. In our study, we, for the very first time, estimated in vivo together—catecholamines (DA and NE) and renalase concentrations. Renalase was significantly higher in our dialyzed patients than in healthy volunteers. What is even more interesting is that it was significantly elevated in the group of anuric and maintained dialysis longer patients. The strong correlation between renalase and residual diuresis was evaluated. Similarly, we indicated a higher NE level in anuric subjects in HD group. This effect is probably caused by much higher SNS activity and much lower renal clearance in that population. No correlation between renalase and BP was found. What is interesting is that, Boomsma and TiptonCitation29 question the method used by Xu et al.Citation6 to measure renalase activity. They considered that the (patho) physiological concentrations of catecholamines were lower than the concentrations used in the experiments. They even concluded that it was unlikely that renalase was a catecholamine-metabolizing enzyme. They suggested that it may have important cardiovascular functions, but through another mechanism. On the other hand, Gu et al.Citation23 found that renalase concentration was higher in heart failure; they suggested that more activated renalase will degrade the increased NE levels. They proposed that the kidney might synthesize and secrete more renalase to compensate the increased catecholamines levels in the early phase of acute myocardial infarction in animal model. In our study, we also indicated higher renalase level in HD patients who died because of cardiac death comparing to those who died because of other causes. The follow up is over 1 year. Therefore, the issue whether renalase is synthesized or just excreted by kidney remains to be elucidated as well as the origin of renalase. Moreover, as DA is associated with regulation of BP, its deficiency leads to hypertension; our findings of significant inverse relationship between renalase and serum DA level in HD group are in line.
The wide use of beta-blockers in our cohort should be also stressed. The beneficial effect of β-blockers in heart failure and CAD in general population is out of debate. There is a controversy in the efficacy of beta-blockers in dialysis patients. Badve et al.Citation30 in the meta-analysis of randomized controlled trials studied the benefits and risk of beta-adrenergic antagonists in patients with CKD stage 3–5 found that treatment with β-blockers decreased all cause and cardiovascular mortality in patients with CKD who had heart failure and low left ventricular ejection fraction. But only one of eight trials included patients undergoing dialysis. In turn, Kitchlu et al.,Citation31 who conducted a retrospective cohort study employing linked healthcare database in Ontario, Canada and studied 1836 chronic dialysis patients, found that β-blockers use was not associated with improved cardiovascular outcomes. In situation when sympathetic overactivity is present like in CKD, β-blocker’s use seems to have a strong pathophysiological rationale.
The limitation of our study is its cross-sectional design, single center, and with rather short follow up. There is no other validated method to assess renalase levels than ELISA assay, however, it was discussed previously.Citation32 The strength of our study is simultaneous measurement of renalase, DA, and noradrenaline in both groups of dialyzed patients: hemodialyzed and peritoneally dialyzed as well as age- and sex-matching of the healthy volunteers. We have demonstrated for the first time that cardiac death was associated with higher renalase. In this regard, follow up presented in our study is a novelty and strengthen the results obtained. The study adds new data on catecholamines and renalase levels in dialyzed patients and their relations to cardiovascular complications, including death.
In conclusions, elevated level of circulating renalase in dialysis patients is rather related to kidney function and the SNS hyperactivity found in this population. The real excess of renalase in the pathogenesis of cardiovascular disorders in patients with CKD still remains to be proven. If confirmed, it may give a new way for pathophysiological therapy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Miller LM, Sood MM, Sood AR, Cardiovascular disease in end-stage renal disease: the challenge of assessing and managing cardiac disease in dialysis patients. Int Urol Nephrol. 2010;42:1007–1014.
- Paraskevas KI, Kotsikoris I, Koupidis SA, Tzovaras AA, Mikhailidis DP. Cardiovascular events in chronic dialysis patients: emphasizing the importance of vascular disease prevention. Int Urol Nephrol. 2010;42:999–1006.
- Zoccali C, Mallamaci F, Parlongo SG, Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359.
- Ritz E, Rump LC. Control of sympathetic activity–new insights; new therapeutic targets? Nephrol Dial Transplant. 2010;25:1048–1050.
- Yoshikawa M, Nakada T. High level of adrenal catecholamines in hypertensive subjects with impaired renal function. Int Urol Nephrol. 1986;18:185–192.
- Xu J, Li G, Wang P, Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005;115:1275–1280.
- Desir GV, Tang L, Wang P, Renalase lowers ambulatory blood pressure by metabolizing circulating adrenaline. J Am Heart Assoc. 2012; 1(4): e002634
- Malyszko J, Zbroch E, Malyszko JS, Koc-Zorawska E, Mysliwiec M. Renalase, a novel regulator of blood pressure, is predicted by kidney function in renal transplant recipients. Transplant Proc. 2011;43:3004–3007.
- Zhao Q, Fan Z, He J, Renalase gene is a novel susceptibility gene for essential hypertension: a two-stage association study in northern Han Chinese population. J Mol Med. 2007;85:877–885.
- Farzaneh-Far R, Desir GV, Na B, Schiller NB, Whooley MA. A functional polymorphism in renalase (Glu37Asp) is associated with cardiac hypertrophy, dysfunction, and ischemia: data from the heart and soul study. PLoS One. 2010;5:e13496.
- K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(Suppl 3):S1–S153.
- Massuo K, Lambert GW. Epinephrine and its role in the development of obesity and hypertension. Curr Hypertens Rev. 2011;7:144–152.
- Massuo K, Esler MD. Role of sympathetic nerve activity in obesity, hypertension and metabolic syndrome. Curr Hypertens Rev. 2010;6:83–91.
- Augustyniak RA, Tuncel M, Zhang W, Toto RD, Victor RG. Symapthetic overactivity as a cause of hypertension in chronic renal failure. J Hypertens. 2002;20:3–9.
- Domingos F, Escalda A. Causes of autonomic dysfunction in idiopathic recurrent kidney stone formers. Int Urol Nephrol. 2012;44:873–882.
- Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728.
- Harris RC, Zhang MZ. Dopamine, the kidney, and hypertension. Curr Hypertens Rep. 2012;14:138–143.
- Harris RC. Abnormalities in renal dopamine signaling and hypertension: the role of GRK4. Curr Opin Nephrol Hypertens. 2012;21:61–65.
- Chen CJ, Apparsundaram S, Lokhandwala MF. Intrarenally produced angiotensin II opposes the natriuretic action of the dopamine-1 receptor agonist fenoldopam in rats. J Pharmacol Exp Ther. 1991;256:486–491.
- Hennebry SC, Eikelis N, Socratous F, Desir G, Lambert G, Schlaich M. Renalase, a novel soluble FAD-dependent protein, is synthesized in the brain and peripheral nerves. Mol Psychiatry. 2010;15:234–236.
- Wu Y, Xu J, Velazquez H, Renalase deficiency aggravates ischemic myocardial damage. Kidney Int. 2011;79:853–860.
- Eikelis N, Hennebry SC, Lambert GW, Schlaich MP. Does renalase degrade catecholamines? Kidney Int. 2011;79:1380.
- Gu R, Lu W, Xie J, Bai J, Xu B. Renalase deficiency in heart failure model of rats–a potential mechanism underlying circulating norepinephrine accumulation. PLoS One. 2011;6:e14633.
- Pandini V, Ciriello F, Tedeschi G, Rossoni G, Zanetti G, Aliverti A. Synthesis of human renalase1 in Escherichia coli and its purification as a FAD-containing holoprotein. Protein Expr Purif. 2010;72:244–253.
- Baroni S, Milani M, Pandini V, Pavesi G, Horner D, Aliverti A. Is renalase a novel player in catecholaminergic signaling? The mystery of the catalytic activity of an intriguing new flavoenzyme. Curr Pharm Des. 2012 Oct 31. [ Epub ahead of print].
- Wang F, Wang N, Xing T, Cao Y, Xiang H. The cloning and expression of renalase and the preparation of its monoclonal antibody. J Shanghai Jiaotong Univ (Sci). 2009;14:376–379.
- Malyszko J, Zbroch E, Malyszko JS, Koc-Zorawska E, Mysliwiec M. Renalase, a novel regulator of blood pressure, is predicted by kidney function in renal transplant recipients. Transplant Proc. 2011;43:3004–3007.
- Przybylowski P, Malyszko J, Kozlowska S, Malyszko J, Koc-Zorawska E, Mysliwiec M. Serum renalase, depends on kidney function but not on blood pressure in heart transplant recipients. Transplant Proc. 2011;43:3888–3891.
- Boomsma F, Tipton KF. Renalase, a catecholamine-metabolising enzyme? J Neural Transm. 2007;114:775–776.
- Badve SV, Roberts MA, Hawley CM, Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:1152–1161.
- Kitchlu A, Clemens K, Gomes T, Beta-blockers and cardiovascular outcomes in dialysis patients: a cohort study in Ontario, Canada. Nephrol Dial Transplant. 2012;27:1591–1598.
- Malyszko J, Malyszko JS, Rysz J, Renalase, hypertension, and kidney – the discussion continues. Angiology. 2012 Sep 11. [ Epub ahead of print].