Abstract
This study aimed at investigating associations between monocytes/macrophages (Mo) infiltration and three important criteria associated with acute antibody-mediated rejection: C4d staining, microcirculation injury, and graft survival time. By quantitative analysis, Mo were counted in peritubular capillaries and in the interstitial compartment (peritubular/interstitial Mo), and they were also identified in glomeruli (glomerular Mo). The study included 47 patients who received renal allograft between 1991 and 2009. Capillaritis and glomerulitis were classified by the Banff scoring system, and C4d and Mo were analyzed by immunohistochemistry. In the quantitative analysis, the mean values of 50 Mo per 10 high-power fields (HPF) and 4 Mo per glomerulus were used as cut-off points for the peritubular/interstitial and glomerular compartments, respectively. Positive C4d cases were associated with the groups of biopsies with a mean value ≥50 Mo per 10 HPF (p = 0.01) and ≥4 Mo per glomerulus (p = 0.02). The group with a mean value ≥4 Mo per glomerulus also showed association with the presence of glomerulitis (p = 0.02). Peritubular/interstitial Mo did not associate with glomerulitis. Capillaritis did not show association with peritubular/interstitial or glomerular Mo. As regards graft survival, the infiltration of Mo in glomeruli interfered with allograft survival (p = 0.01). The group with a mean value of ≥4 glomerular Mo presented worse survival at the time of the 1-year follow-up. According to the literature, our data showed that infiltration of mononuclear cells was associated with C4d staining, microcirculation injury, and glomerulitis, in particular, and that glomerular macrophages could influence renal allograft survival.
INTRODUCTION
Monocytes/macrophages (Mo) have shown fundamental relevance for the prognosis of kidney-transplant patients. Large numbers of macrophages comprising more than 50% of inflammatory cells are usually recognized in severe acute rejection cases.Citation1 Studies have also considered that their presence may unfavorably affect clinical outcomes.Citation2
The earliest investigations on Mo had already shown that these cells participated in acutely rejecting renal allograft.Citation3,Citation4
Hancock et al. (1983) and Reitamo et al. (1980) reported large numbers of interstitial macrophages in acute rejection (38–60% of infiltrating leukocytes). The largest percentage was observed in cases of severe acute rejection, usually in peritubular and glomerular locations.Citation5,Citation6
Antibody-mediated rejection (AMR) has been known for decades, but its significance in acute rejection has only recently been recognized.Citation7,Citation8 Currently, one of the major obstacles to the recognition of this type of rejection has been the lack of other reliable immunologic tissue markers in addition to C4d.Citation9 The nature of inflammatory cells is especially important for an accurate diagnosis of acute AMR. Some evidence suggests that the infiltration of macrophages in glomeruli and peritubular capillaries is associated with acute AMR and C4d.Citation10,Citation11
Recently, attention has been given to injury in the graft microcirculation as an important factor responsible for disease progression in kidney rejections.Citation12 Injury in the renal microvasculature results in tissue hypoxia, promoting progression of renal damage and scarring.Citation13
Some studies have shown endothelial cell injury and significant loss of peritubular capillaries associated with macrophage infiltration in the end stage of chronic allograft failure.Citation14,Citation15 A large amount of Mo infiltration, especially in glomeruli, has also been shown to be indicative of poor graft outcomes.Citation16
As effectors of tissue injury, Mo secretes a large number of proteolytic enzymes and reactive oxygen species. Mo is also responsible for secreting a wide variety of proinflammatory cytokines and growth factors that promote tissue damage and progression to sclerosis.Citation17–19
The aim of our study was to quantify, by immunohistochemistry, all intracapillary, interstitial, and glomerular Mo in biopsies with acute rejection and to show associations between Mo and peritubular capillaritis or glomerulitis, C4d staining, and allograft survival.
PATIENTS AND METHODS
We used the Banff 07 criteria to retrospectively analyze 47 biopsies clinically diagnosed as acute rejection between January 1991 and March 2009.
Only the first post-transplant renal biopsy performed because of acute functional impairment with diagnostic of acute rejection was included in the study. Twelve biopsies were obtained during the first week, 12 during the first month, 10 between the first and third months, and 13 were obtained 3 months following transplantation.
Human leukocyte antigen (HLA) matching was defined as the number of HLA-matched antigens at loci A, B, and DR. All transplant recipients exhibited a negative pretransplant cytotoxic cross match, as tested against peripheral blood lymphocytes from living donors or lymphocytes from the lymph nodes or spleen from deceased donors. Pretransplant panel-reactive antibodies (PRAs) were determined by using the Luminex Assay before transplantation. Delayed graft function was defined as the need for dialysis within the first week post-transplantation. Graft loss was defined as the resumption of dialysis, and survival was censored for patients dying with functional grafts.
The presence of donor-specific antibodies (DSA) could not be shown because some cases were ancient and had incomplete information.
Initial immunosuppression was achieved by using different types of therapeutic regimens (usually a triple therapy regimen). Percentage of using azathioprine (1.0–1.5 mg/kg/day), 42.5%; prednisone (30 mg/day), 100%; calcineurin inhibitors—cyclosporine (7.0 mg/kg/day) or tacrolimus (0.2 mg/kg/day), 93.6%; mycophenolate mofetil (2.0 mg/day), 57.4%; basiliximab (20 mg first and the fourth postoperative period), 44.7%; thymoglobulin (1.0 mg/kg/day for 10 days), 2%; and mTOR inhibitor, 2%. During episode of acute rejection, the following were used as treatment: methylprednisolone in 89.3% of the cases, OKT3 in 19%, and thymoglobulin in 8.5%. Plasmapheresis or intravenous immune globulin was not used in any patient.
Detailed clinical data were obtained via retrospective chart reviews ().
Table 1. Description of clinical features.
The study was approved by the Research Ethics Committee of the Botucatu School of Medicine (Of. 511/08).
HISTOPATHOLOGICAL ANALYSIS
Histopathological evaluation was performed on Duboscq-Brazil-fixed paraffin-embedded 3-µm tissue sections stained with hematoxylin and eosin, periodic acid-Schiff, methenamine-silver, and Masson trichrome. Frozen kidney tissue was analyzed by the immunofluorescence technique using mono-specific mouse antisera against human IgG, IgM, IgA, C1q, C3, and fibrinogen (Dako, CA/USA).
Allograft biopsies were classified according to the Banff classification criteria. The scoring of interstitial inflammation (i), tubulitis (t), arteritis (v) was graded; vascular thrombosis and areas of hemorrhage and necrosis were also noted.Citation20
Peritubular capillaritis (ptc) and glomerulitis scores were assessed using the Banff scoring system.Citation20,Citation21
IMMUNOHISTOCHEMISTRY
Immunohistochemical staining was performed on 47 biopsies for anti-human CD68 KP1 mouse monoclonal antibodies (Dako Inc., CA, USA) and for anti-human IgG C4d rabbit polyclonal antibodies (BI-RC4d-Biomedica, Vienna/Austria).
For immunohistochemical studies, 3-µm tissue sections were mounted on silanated slides, dried for 15 h at 58˚C, and deparaffinized with three xylene washes (5 min each), hydrated in absolute alcohol and washed in distilled water. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. Antigen retrieval was performed by pressure cooking for 3 min with EDTA, pH 8.1 for CD68, and in citrate buffer, 10 mM, pH 6.0 for C4d. After washing in distilled water and phosphate-buffered saline (PBS), the sections were incubated with primary antibodies for 30 min at room temperature: anti-CD68 was diluted at 1:800 and anti-human C4d was diluted at 1:50. After the final PBS wash, the slides were incubated with the polymer reagent (EnVision-Dako Inc., CA, USA) for 30 min at room temperature and washed in PBS. The reaction was visualized with diaminobenzidine (Dako Inc., CA, USA). Counterstaining was performed with 50% Harris hematoxylin, and after washing in water, the sections were mounted with entellan.
Thirty renal allograft biopsies taken at the time of transplantation (zero-time baseline biopsies of the studied patients) were used as controls.
The intensity of C4d staining in peritubular capillaries was evaluated by determining the percentage of C4d+ capillaries in 10 high-power fields (HPF) (400× magnification), and at least 20 capillaries were counted. Areas of necrosis and fibrosis were excluded.
Overall, C4d staining was considered to be negative when less than 1% of capillaries was stained positive, indeterminate when 1–10% of capillaries was C4d+ , focal positive when 10–50% of capillaries were positive, or diffuse positive when more than 50% of capillaries were stained.
QUANTITATIVE ANALYSIS OF MONOCYTES/MACROPHAGES
The quantitative analysis of Mo in the interstitium and in peritubular capillaries (peritubular/interstitial Mo) was determined by counting the number of CD68+ cells in the interstitium and in peritubular capillaries in 10 HPF. The analysis of glomerular Mo was determined by counting the number of CD68+ cells in all glomeruli, and it was expressed as the mean value of infiltrating glomerular Mo (gMo) per biopsy.
Groups of study
Group 1. Mean peritubular/interstitial Mo less than 50 (<50 Mo) and equal to or more than 50 (≥50 Mo) per HPF.
Group 2. Mean glomerular Mo less than 4 (<4 gMo) and equal to or more than 4 (≥4 gMo) per glomerulus.
Statistical Analysis
Data are reported as the mean ± standard deviation or as the median with ranges.
The association of categorical variables was analyzed by using chi-square tests (χ2) and Fisher’s exact tests, when necessary.
All statistical calculations were performed using SAS software (Windows, version 9.2, SAS Inc., Cary, NC, USA).
Graft survival was calculated by the Kaplan–Meier method and the log-rank test using the GraphPad Prism software (version 5.05, GraphPad Prism Software Inc., San Diego, CA, USA). p-Values <0.05 were considered to be statistically significant.
RESULTS
In the quantitative analysis of peritubular/interstitial Mo (group 1), 22 cases (46.8%) showed <50 Mo per 10 HPF and 25 cases (53.2%) showed ≥50 Mo per 10 HPF per biopsy. As regards the glomeruli (group 2), 28 cases (59.6%) showed a mean value <4 gMo per biopsy and 19 cases (40.4%) showed ≥4 gMo per biopsy.
Sixteen cases (34.1%) showed only tubulointerstitial inflammation, and 31 cases (approximately 65.9%) showed arteritis associated with tubulointerstitial inflammation. Glomerular thrombosis, interstitial hemorrhage, and infarction were detected in 6.3%, 8.5%, and 6.3% of cases, respectively. Biopsies revealed that the majority of infarction cases were also characterized by vascular lesions.
There was only statistically significant difference between arteritis and group 2 (p = 0.002). A larger number of cases with arteritis were observed in the group, showing a mean value ≥4 gMo per biopsy.
In the score for ptc, 13 cases (27.6%) were classified as ptc0 or ptc1 and 34 cases (72.4%) were classified as ptc2 or ptc3. There was no statistically significant difference between peritubular capillaritis and group 1 or 2.
In relation to glomerulitis (g), 38 cases (80.8%) were classified as g0 or g1 and 9 (approximately 19.2%) cases as g2 or g3. Statistically significant difference was observed between glomerulitis and group 2 (p = 0.02). Biopsies G2 + G3 were predominantly observed in the cases with ≥4 gMo per biopsy. No significant association was observed between glomerulitis and group 1.
In 47 biopsies, the distribution of C4d staining along peritubular capillaries was diffuse positive (C4d3) in 5 (10.6%) cases, focal positive (C4d2) in 10 (21.2%), indeterminate (C4d1) in 12 (25.5%), and negative (C4d0) in 20 (42.6%) cases.
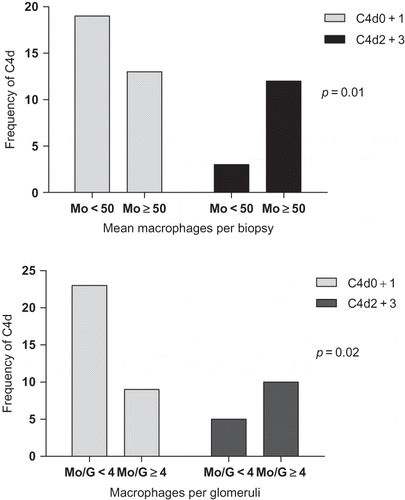
There was statistically significant difference between group 1 and C4d (p = 0.01) and group 2 and C4d (p = 0.02). Positive C4d cases (C4d2 + C4d3) were predominant in the biopsies with ≥50 Mo HPF and ≥4 gMo ().
Figure 1. Associations between C4d staining and peritubular/interstitial (A) and glomerular (B) monocytes/macrophages.
No significant associations were observed between Mo infiltration and clinical features. describes the frequencies of mismatches and PRA to the groups.
Table 2. Pretransplant panel-reactive antibodies (PRA) and number of mismatches to each group.
Graft Survival
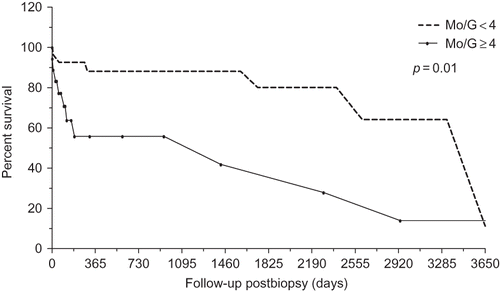
During the follow-up period established in this study (46.9 ± 46.1 months), 31% of patients lost their allograft.
Biopsies characterized by group 1 showed a tendency to statistical significant difference for graft survival time (p = 0.05). However, biopsies characterized by group 2 showed statistically significant difference in relation to graft survival time (p = 0.01). At the time of the 1-year follow-up, graft survival for the group with <4 gMo was approximately 90%, but approximately 55.8% for the group with ≥4 gMo ().
DISCUSSION
In studies on transplant immunology, there is evidence that the innate immune system plays an important role in allograft injury. Macrophages are cells that participate in the innate immune response, and some studies suggest that macrophages can promote acute allograft rejection.Citation22–24 The presence of macrophages infiltration is considered to be important for prognosis in human renal transplantation.Citation25 Studies have shown that macrophages participate in the tubulointerstitial injury by producing nitric oxide.Citation26 This mechanism can lead to loss of the renal microvasculature, tissue hypoxia, renal injury, and scarring.Citation13 Each episode of acute rejection is accompanied by loss of peritubular microvasculature, and without microvascular regeneration, this would lead to worse graft survival.Citation27
Our study does not discriminate between intravascular and interstitial monocytes. It is usually difficult to distinguish between cells within peritubular capillaries and extracapillary inflammatory cells by conventional histology or by immunohistochemical stains for inflammatory cells.Citation28
This study showed associations between mononuclear cell infiltration in C4d-positive cases and microvasculature injury. Such association was observed both in mononuclear interstitial/peritubular infiltration and in mononuclear glomerular infiltration.
The recruitment and activation of monocytes might enhance the inflammatory reaction initiated by antibody-binding and -complement activation, and they can contribute to microvascular injury in glomeruli and peritubular capillaries.Citation10 According to our results, Tinckam et al. (2005) also demonstrated that glomerular macrophage counts had an unfavorable impact on outcomes and that a predominant infiltration of endocapillary cells composed by macrophages was associated with C4d+ .Citation29
Additionally, there is evidence that peritubular capillaritis is associated with graft survival30 and that glomerulitis is associated with peritubular capillary C4d staining and graft loss.Citation23
Our study showed a significant association between the accumulation of monocytes within glomeruli and graft survival time. Accumulation of mononuclear cells in the peritubular capillaries and interstitium did not show the same survival outcome.
It seems that glomeruli and the interstitium are distinct immunologic compartments. Phagocytic cells, such as macrophages, representing innate immunity, would act mainly in the glomeruli, and adaptive immunity would predominate in the interstitium.Citation31 This probability could also explain the positive associations between macrophage glomerular infiltration and graft survival. Glomerular macrophages can be an independent prognostic factor in spite of also being associated with other morphological features related to a worse prognosis.
In conclusion, this study showed, according to the literature, that infiltrating mononuclear cells are associated with microcirculation injury, particularly with glomerulitis, and C4d staining in acute rejection and that glomerular macrophages in acute rejection showed a correlation to allograft survival in kidney transplantation.
ACKNOWLEDGMENTS
The authors thank Dr. Daísa Silva Ribeiro David for her teaching assistance.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Häyry P, Von Willebrand E. Monitoring of human renal allograft rejection with fine-needle aspiration cytology. Scand J Immunol. 1981;13:87–97.
- Kozakowski N, Böhmig GA, Exner M, Monocytes/macrophages in kidney allograft intimal arteritis: no association with markers of humoral rejection or with inferior outcome. Nephrol Dial Transplant. 2009;24(6):1979–1986.
- Strom TB, Tilney NL, Carpenter CB, Identity and cytotoxic capacity of cells infiltrating renal allografts. N Engl J Med. 1975;292:1257–1263.
- Von Willebrand E, Häyry P. Composition and in vitro cytotoxicity of cellular infiltrates in rejecting human kidney allografts. Cell Immunol. 1978;41:358–372.
- Hancock WW, Thomson NM, Atkins RC. Composition of interstitial cellular infiltrate identified by monoclonal antibodies in renal biopsies of rejecting human allografts. Transplantation. 1983;35:458–463.
- Reitamo S, Konttinen T, Ranki A, The relation of different inflammatory cell types to the various parenchymal components of rejecting kidney allograft. Histopathology. 1980;4:517–532.
- Jeannet M, Pinn VW, Flax MH, Humoral antibodies in renal allotransplantation in man. N Engl J Med. 1970;282:111–117.
- Halloran PF, Wadgymar A, Ritchie S, Falk J, Solez K, Srinivasa NS. The significance of anti-class I antibody response. Clinical and pathologic features of anti-class I mediated rejection. Transplantation. 1990;49:85–91.
- Haas M. C4d-negative antibody-mediated rejection in renal allografts: evidence for its existence and effect on graft survival. Clin Nephrol. 2011;75(4):271–278.
- Sund S, Reisaeter AV, Scott H, Mollnes TE, Hovig T. Glomerular monocyte/macrophage influx correlates strongly with complement activation in 1-week protocol kidney allograft biopsies. Clin Nephrol. August 2004;62(2):121–130.
- Fahim T, Böhmig GA, Exner M, The cellular lesion of humoral rejection: predominant recruitment of monocytes to peritubular and glomerular capillaries. Am J Transplant. February 2007;7(2):385–393.
- Hall AV, Jevnikar AM. Significance of endothelial cell survival programs for renal transplantation. Am J Kidney Dis. 2003;41:1140.
- Fine LG, Orphanides C, Norman JT. Progressive renal disease. The chronic hypoxia hypothesis. Kidney Int Suppl. 1998;65:S74.
- Adair A, Mitchell DR, Kipari T, Peritubular capillary rarefaction and lymphangiogenesis in chronic allograft failure. Transplantation. 2007;83:1542.
- Qi F, Adair A, Ferenbach D, Vass DG, Depletion of cells of monocyte lineage prevents loss of renal microvasculature in murine kidney transplantation. Transplantation. 2008;86:1267–1274.
- Magil AB. Monocytes/macrophages in renal allograft rejection. Transplant Rev. 2009;23(4):199–208.
- Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–326.
- Baricos WH, Shah SV. Proteolytic enzymes as mediators of glomerular injury. Kidney Int. 1991;40:161–173.
- Cattell V. Macrophages in acute glomerular inflammation. Kidney Int. 1994;45:945–952.
- Racusen LC, Solez K, Colvin RB, The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723.
- Solez K, Colvin RB, Racusen LC, Banff 05 meeting report: differential diagnosis of chronic allograft injury and elimination of Chronic Allograft Nephropathy (‘CAN’). Am J Transplant. 2007;7:518.
- Trpkov K, Campbell P, Pazderka F, Cockfield S, Solez K, Halloran PF. Pathologic features of acute renal allograft rejection associated with donor-specific antibody. Analysis using the Banff grading schema. Transplantation. June 15, 1996;61(11): 1586–1592.
- Mauiyyedi S, Crespo M, Collins AB, Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology and pathologic classification. J Am Soc Nephrol. 2002;13:779–787.
- Solez K, Colvin RB, Racusen LC, Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760.
- Regele H, Exner M, Watschinger B, Endothelial C4d deposition is associated with inferior kidney allograft outcome independently of cellular rejection. Nephrol Dial Transplant. 2001;16:2058–2066.
- Kipari T, Cailhier JF, Ferenbach D, Nitric oxide is an important mediator of renal tubular epithelial cell death in vitro and in murino experimental hidronephrosis. Am J Transplant. 2006;169:388.
- Land WG. Innate immunity-mediated allograft rejection and strategies to prevent it. Transplant Proc. 2007;39:667.
- Fahim T, Böhmig GA, Exner M, The cellular lesion of humoral rejection predominant recruitment of monocytes to peritubular and glomerular capillaries. Am J Transplant, 2007;7:385.
- Tinckam KJ, Djurdjev O, Magil AB. Glomerular monocytes predict worse outcomes after acute renal allograft rejection independent of C4d status. Kidney Int, 2005;608:1866.
- Cosio FG, Lager DJ, Lorenz EC, Significance and implications of capillaritis during acute rejection of kidney allograft. Transplantation. 2010;89:1088.
- Sereger S, Heller F, Lindenmeyer MT, Compartment specific expression of dentritic cell markers in human glomerulonephritis. Kidney Int. 2008;74:37.