Abstract
Introduction: Acetaminophen (APAP) is an analgesic and antipyretic agent. In overdoses, it is associated with nephrotoxicity. We examined the potential protective effects of N-acetylcysteine (NAC) and NAC + ozone therapy (OT) combination against APAP-induced nephrotoxicity. Materials and methods: Thirty-two male Sprague–Dawley rats were divided into four groups: sham, control (APAP), NAC, and NAC + OT. In the APAP, NAC, and NAC + OT groups, kidney injury was induced by oral administration of 1 g/kg APAP. The NAC group received NAC (100 mg/kg/day). NAC + OT group received NAC (100 mg/kg/day) and ozone/oxygen mixture (0.7 mg/kg/day) intraperitoneally for 5 days immediately after APAP administration. All animals were killed at 5 days after APAP administration. Renal tissues and blood samples were obtained for biochemical and histopathological analyses. Neopterin, tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and IL-10 levels were measured in sera. Malondialdehyde (MDA) levels and glutathione peroxidase (GPx) activities were determined in renal homogenates. Results: NAC and NAC + OT significantly decreased MDA and TNF-α levels and increased IL-10 levels and GPx activities. Serum neopterin and IL-6 levels were not different among all groups. APAP administration caused tubular necrosis in the kidney. The degrees of renal necrosis of the APAP group were higher than the other groups. Renal injury in rats treated with combination of NAC and OT were found to be significantly less than the other groups. Conclusions: Our results showed that NAC and OT prevented renal injury in rats and reduced inflammation. These findings suggest that combination of NAC and OT might improve renal damages because of both oxidative stress and inflammation.
INTRODUCTION
The analgesic and antipyretic agent acetaminophen (N-acetyl-p-aminophenol, APAP) is widely used in adults and children. It has a safety profile when administered in therapeutic doses.Citation1 Although highly effective, it is associated with hepatotoxicity and nephrotoxicity in both humans and animals if taken in overdose.Citation1
APAP is metabolized to the reactive derivative N-acetyl-p-benzquinamide (NAPQI) by cytochrome P450 enzyme.Citation1,2 At therapeutic doses, this metabolite is efficiently detoxified by being conjugated with glutathione (GSH). At overdoses, however, APAP leads to accumulation of NAPQICitation1 and critical depletion of GSH.Citation3 The mechanism of APAP-induced nephrotoxicity is not entirely known. It is thought to be due to oxidative stress with a critical depletion of GSH and renal inflammation.Citation4
Neopterin, a guanosine triphosphate metabolite, is produced and releases macrophages and monocytes in response to stimulation by interferon-γ and other cytokines.Citation5–8 In addition, neopterin is known as an indicator for severity of some diseases.Citation5–9
N-Acetylcysteine (NAC) is widely used in both children and adults for treatment of acetaminophen overdose. NAC serves as a GSH precursor, increasing its synthesis and refilling its pools.Citation3 Its characteristics suggest that NAC is a potentially effective candidate for renal protection against APAP-induced toxicity. Although NAC protects against APAP hepatotoxicity, it is unable to protect against APAP nephrotoxicity.Citation10 Kidneys cannot synthesize GSH and have to take from circulation their need, while liver can synthesize GSH from NAC.Citation11 On the other hand, it has also been shown to have a protective effect on the kidney in experimental studies.Citation3
Administration of a gas mixture comprising ozone/oxygen (O3/O2) is known as ozone therapy (OT).Citation12 O3/O2 mixture has various effects on the immune system and antioxidant features.Citation9,13 It was previously reported that OT increased antioxidant enzyme activities such as glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT).Citation14 It was also demonstrated that OT altered levels of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) Citation15 and interferon-γ.Citation16
It is important to improve new treatment approaches against APAP-induced nephrotoxicity. In this study, we examined the potential protective effects of NAC and NAC + OT combination against APAP-induced nephrotoxicity.
MATERIAL AND METHODS
Animals and Study Groups
All procedures involving the use of laboratory animals were reviewed and approved by the Animal Ethics Committee of Gulhane Military Medical Academy. Thirty-two male Sprague–Dawley rats (230–250 g) were obtained from Kobay Deney Hayvanlari Laboratuvari A.S. Yenimahalle, Ankara, Turkey. The animals were housed in a controlled environment with room temperature and a 12-h light–dark cycle. Animals were fed with standard rat chow and fresh water ad libitum for a week before experiments. Rats were randomly divided into four groups containing eight animals each: sham, control (APAP treated only), APAP + NAC therapy (NAC), and ozone + N-acetylcysteine (APAP + OT + NAC) (NAC + OT) groups.
Induction of Acetaminophen Toxicity
APAP (1.0 g/kg body weight) (Ordu Ilac Fabrikasi, Ankara, Turkey) was suspended in hot distilled water (3 mL, at 50°C). A suspension of APAP was administered (at 25°C) to rats in control, NAC, NAC + OT groups at a single dose of APAP by gastric tube. Rats in the sham group received only distilled water (at 25°C) by gastric tube.
Treatments
Immediately after the administration of single-dose APAP, the rats in the NAC + OT group were administrated O3/O2 mixture at a dose of 0.7 mg/kg (once daily) intraperitoneally for 5 days. O3 was generated by the ozone generator (OZONOSAN Photonik 1014, Hansler GmbH, Nordring 8, Iffezheim, Germany), allowing control of the gas flow rate and O3 concentration in real time by a built-in UV spectrometer. The O3 flow rate was kept constant at 3 L/min, representing concentration of 60 mg/mL and gas mixture of 97% O2 + 3% O3. Tygon polymer tubes and single-use silicon-treated polypropylene syringes (ozone resistant) were used throughout the reaction to ensure containment of O3 and consistency of concentrations.
Immediately after the administration of single-dose APAP, the rats in the NAC and NAC + OT groups were administrated NAC at a dose of 100 mg/kg (once daily) intraperitoneally for 5 days.
Sample Preparation
On day 6, after APAP treatment, all animals were killed under light diethyl ether anesthesia. Blood samples were collected via cardiac puncture for the measurement of biochemical parameters before the animals were killed. Both kidneys were harvested and cleaned. For histological studies, renal tissue samples were stored in 10% formalin solution. The remaining renal tissues were immediately frozen in liquid nitrogen and stored in a deep freezer at −80°C until further analysis. The frozen tissues were homogenized in phosphate buffer (pH 7.4) by means of homogenization (Heidolph Diax 900, Heidolph Elektro GmbH, Kelhaim, Germany) on an ice cube. The supernatant was used for the entire assays. The protein content of renal homogenates was measured by the method of Lowry et al. using bovine serum albumin as the standard.Citation17
Biochemical Analysis
Serum urea and creatinine levels were measured with a spectrophotometric technique by the Olympus AU-2700 autoanalyzer using commercial kits (Olympus, Hamburg, Germany) and presented as milligram per deciliter (mg/dL).
The serum TNF-α, interleukin (IL)-6 and IL-10 levels were measured by enzyme-linked immunosorbent assay kits (Bender MedSystems GmbH, Vienna, Austria).
GPx activity was measured using the method described by Paglia and Valentine.Citation18 GPx activity was coupled with the oxidation of NADPH by GSH reductase. The oxidation of NADPH was spectrophotometrically followed at 340 nm and 37°C, and the absorbance was recorded for 5 min. The activity was the slope of the lines as mmol of NADPH oxidized per minute. GPx activity was presented as U/g protein.
Malondialdehyde (MDA) was determined based on the production of thiobarbituric acid reactive substances in the supernatant by the method described by Al-Fawaeir et al.Citation19 After the reaction of MDA with thiobarbituric acid, the reaction product was followed spectrophotometrically at 532 nm, using tetrametoxypropane as a standard. The results were expressed as nmol/mg protein.
Serum neopterin levels were measured with a high performance liquid chromatography device (Agilent Technologies 1200 Series System, Santa Clara, CA, USA), using the method defined by Agilli et al.Citation20 In brief, to 0.1 mL serum, 0.1 mL 100% acetonitrile was added and vortexed. The precipitated protein was removed by centrifugation at 4°C and 10,000g for 10 min. Hundred microliter of the supernatant was filtered through a 0.2 μm filter and then injected into the chromatographic system. Separation of neopterin was achieved with a 150 × 4.6 mm I.D. Hichrom ODS-3, C18 RP column with a particle size of 5 μm (Hichrom Limited, Berks, UK) fitted with a Phenomenex C18, 5 μm guard column (Phenomenex, Cheshire, UK) using water/acetonitrile (99/1, v/v) as mobile phase (isocratic elution) at a flow rate of 1.0 mL/min. The areas of peaks detected by fluorescent detector (Ex: 353 nm; Em: 438 nm) were used for quantification. Serum neopterin levels were expressed as nmol/L.
Histological Analysis
Examination of kidney histology was performed according to routine histology techniques. This processing consisted of specimens’ fixation in 4% buffered neutral formalin solution for 24 h, embedding in paraffin wax, slicing cut sections at 4–5 μm of thickness, and staining them with hematoxylin and eosin (H&E). Histopathological examination was blindly evaluated by a nephropathologist. All sections of kidney samples were examined for characteristic histological changes including glomerular, tubular, interstitial, and vascular alterations.
Statistical Analysis
All the statistical analyses were performed by using SPSS 15.0 software (SPSS Inc., Chicago, IL, USA). Distributions were evaluated by using one-sample Kolmogorov–Smirnov test to determine whether the continuous variables are normally distributed or not. Then, we used Kruskal–Wallis test for the variables that are not normally distributed. For the pairwise comparisons, we used Bonferroni-corrected Mann–Whitney U-test with respect to distribution. χCitation2-Test was used for comparing the grades of tubular injury. The results were expressed as median (min–max). A p-value <0.05 was considered statistically significant for overall comparisons and p < 0.0083 for Bonferroni-corrected pairwise comparisons.
RESULTS
No deaths were observed in any groups of rats. All laboratory parameters that belong to all experimental groups are shown in . After treatment with APAP, serum creatinine levels were not different among all groups (p = 0.779). Also, there was no statistically significant difference among all groups in serum urea levels (p = 0.647). There was no difference among all groups in terms of serum neopterin and IL-6 levels (p = 0.451 and p = 0.747, respectively).
Table 1. Biochemical findings in the all experimental groups.
The concentrations of serum TNF-α in the sham group [88 (84–99) pg/mL] were significantly different compared with the control [159 (142–172) pg/mL], NAC [125.5 (120–199) pg/mL] and NAC + OT [104.5 (92–176) pg/mL] groups (p < 0.001, p = 0.004 and p = 0.037, respectively). But there was no difference between the control and the NAC and the NAC + OT groups (p = 0.068 and p = 0.069, respectively). On the other hand, there was no difference between the NAC and the NAC + OT groups (p = 0.273).
The lowest serum IL-10 concentrations [22.3 (21.0–25.6) pg/mL] were also in the sham group. These concentrations were significantly different than those of the control [28.1 (24.1–31.7) pg/mL], NAC [33.6 (29.4–38.6) pg/mL], and NAC + OT [48.5 (33.6–102.1) pg/mL] groups (p < 0.001, p = 0.005 and p = 0.005, respectively). In the control group, IL-10 levels were significantly lower than in the NAC and the NAC + OT groups (p = 0.011 and p = 0.005, respectively). In the NAC group, serum IL-10 concentrations were lower than in the NAC + OT group, but it was not statistically significant (p = 0.092).
Following administration of APAP to rats, renal MDA levels were significantly increased in the control group. But there was significant decrement in antioxidant GPx enzyme activities in control group. Treatment of the rats with NAC and NAC + OT resulted in a significant decrease in renal MDA levels (in both, p = 0.005). But the increment in the tissue GPx activities was not significant compared to control groups (p = 0.691 and p = 0.070, respectively).
All histopathologic changes were scored as described in . Light microscopic examination of the kidneys revealed obvious mild to moderate glomerular congestion and prominent tubular injury, which consisted of epithelial necrosis, epithelium with necrotic luminal debris, diminished brush borders, vacuolization, tubular necrosis, moderate to severe interstitial edema, peritubular capillary congestion, and inflammation in the control group (A) compared with the sham group (B). The highest histopathologic scores were in the control group. These scores were significantly different than those in the other groups (p < 0.001, ). But there was no difference in terms of vascular changes. Tubular injury was distinct (A). In the NAC and NAC + OT groups, glomerular, tubular, and interstitial changes were better than in the control group (C and D). In the NAC and the NAC + OT groups, histopathologic scores were significantly lower than in the control group (in both, p < 0.001). In the NAC and NAC + OT groups, glomerular changes were almost similar to those in the sham group. In the NAC + OT group, histopathologic scores were lower than in the NAC group, but it was not statistically significant (p = 0.794).
Figure 1. (A) A case in the control (APAP) group demonstrates severely injured tubular and interstitial structures (epithelial necrosis, attenuated epithelium with necrotic luminal debris, diminished brush border) and mild to moderate interstitial edema (H&E, ×200). (B) All structures of kidney show histopathologically normal kidney tissue in the sham group (H&E, ×200). (C) A case in the NAC group shows mild tubular injury (epithelial vacuolization) and minimal interstitial edema (H&E, ×200). (D) In the NAC + OT group, most of tubular and interstitial areas are normal (H&E, ×200).
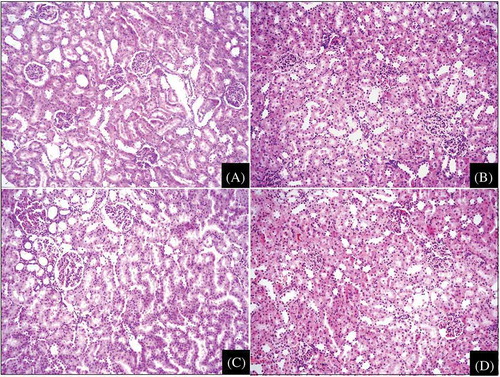
Table 2. Mean scores of histopathologic changes in all animal groups.
DISCUSSION
Our study showed that administration of 1 g/kg APAP to rats resulted in significant histopathologic changes in kidney. In addition, APAP caused oxidative stress in renal tissues. After APAP administration to rats, NAC and NAC + OT reduced histopathologic changes, oxidative stress, and inflammatory parameters in this study.
In the overdoses, APAP causes hepatic and renal injury in both human and experimental animals.Citation6,21,22 Recent studies suggest that oxidative stress and inflammation have an important pathophysiological role in cisplatin-, ifosfamide-, APAP-induced renal toxicities.Citation13,23–27 In particular, TNF-α plays an important role in cisplatin-induced injury.Citation26 Many studies suggest that pro-inflammatory cytokines have an important role in pathogenesis of APAP-induced liver toxicity.Citation6 On the contrary, anti-inflammatory cytokines have protective roles against APAP toxicity.Citation6 According to our knowledge, physiopathological mechanisms of APAP-induced toxicity are well described in the liver but are clearly less understood in the kidney. It has been demonstrated that APAP administration leads to activation of nuclear factor-kappa B (NF-κB) in tubular cells and finally causes renal inflammation.Citation4 NF-κB regulates the expression of various genes, including IL-1β, TNF-α that plays critical roles in inflammation.Citation4
Lipid peroxidation may play a causative role or may only contribute to the pathogenesis of APAP-induced renal injury.Citation11,13,23 Li et al. have suggested that lipid peroxidation plays a role in APAP-induced renal injury.Citation28 It has been reported that intracellular GSH plays a major role in the prevention of APAP-induced nephrotoxicity.Citation4,13 An important decrement in renal GSH concentrations and GPx activities are shown in the APAP-induced nephrotoxicity.Citation4,13,21 This may be due to change of APAP to the reactive metabolite, NAPQI.Citation29 APAP-induced nephrotoxicity results from NAPQI, which arylates proteins in the proximal tubule and initiates the death of renal tubular cells. And, this is frequently associated with acute tubular necrosis.Citation21 On the other hand, accumulation of NAPQI depletes liver GSH levels and causes mitochondrial dysfunction and acute hepatic necrosis.Citation1 In addition, NAPQI also leads to depletion of intracellular GSH levels in the kidney.Citation30 Decreased renal GSH levels allow lipid peroxidation. It is closely related to APAP tissue damage.Citation29 Our results have shown that administration of 1 g/kg APAP caused significant elevations in renal MDA levels in the control group compared to the sham group. In our study, MDA levels were significantly decreased in the kidneys of rats treated with NAC and NAC + OT. It has been suggested that APAP-induced tissue injury is closely related to lipid peroxidation. Our results are consistent with those of other studies.Citation2,31,32 We demonstrated that the administration of APAP-induced oxidative stress in the kidney. This was demonstrated with increased lipid peroxidation and decreased GPx enzyme activities. Increased MDA levels in renal tissues have been shown in animal models of APAP-induced nephrotoxicity.Citation13,23
NAC is known as a free radical scavengerCitation32 and may replenish decreased tissue GSH stores.Citation25,33 NAC has two important mechanisms to protect tissues from oxidative stress. It is a precursor for GSH synthesis and a scavenger for reactive oxygen species.Citation25,32 Chen et al. demonstrated the protective effects of NAC in acute ifosfamide-induced nephrotoxicity.Citation25 They have reported that NAC protects the renal cells from depletion of GSH, lipid peroxidation, and histopathological changes in kidney.Citation25 Although NAC increases intracellular GSH levels, it cannot show sufficient protection against APAP-induced renal injury. The reason for this may be that the actual GSH producer is the liver rather than the kidneys. Because the kidneys cannot produce, they have to take sufficient GSH from circulation.Citation11 Similarly, in this study, administration of NAC and NAC + OT also decreased MDA levels and increased GPx activities of renal tissue. It has been reported that OT decreased renal MDA levels and significantly increased renal GPx enzyme activities in an APAP-induced nephrotoxicity model.Citation13 Our results agreed with this study. Beneficial effect of OT may be eliminating or reducing oxidative stress occurring in APAP-induced nephrotoxicity.
Various studies have shown that OT has different effects on the immune system and oxidative stress.Citation5,9,13,34–37 OT decreases lipid peroxidation and inflammation, whereas increases antioxidant enzyme activities such as GPx, SOD, and CAT.Citation37 Our results have demonstrated that APAP treatment leads to inflammation in the renal tissue. The control group rats had elevated serum TNF-α levels. This is in agreement with the study performed by Fouad et al.Citation38 NAC and NAC + OT also diminished serum TNF-α, a pro-inflammatory cytokine, concentrations and increased serum IL-10, an anti-inflammatory cytokine, concentrations after APAP treatment. But NAC + OT administration significantly decreased inflammation in the injured kidneys through anti-inflammatory and antioxidant mechanisms. In addition, NAC + OT showed a meaningful protection against APAP-induced renal injury. But protection of NAC was weaker. Neopterin has become one of the useful tools to assess the intensity of the cell-mediated immune response. Plasma or urine neopterin concentrations reflect the activation of the macrophage/monocyte line cells.Citation39 In this study, serum neopterin and IL-6 levels were not significantly altered in the APAP-treated rats compared to the sham group. On the other hand, Cakir et al. have reported that APAP-induced renal toxicity causes a dramatic elevation in tissue neopterin levels.Citation6 And also, OT significantly decreases serum neopterin levels in a renal ischemia/reperfusion model.Citation37
Following the administration of APAP, we observed that the histopathological changes of renal toxicity were obvious evidences. These results of histopathological examination agree with those of the other investigations describing after-overdose APAP treatment.Citation6,28,40,41 However, APAP-induced renal injury was not consistent with serum creatinine and urea levels. These results may be due to low dose of APAP. In this study, APAP caused tubular vacuolization, tubular necrosis, interstitial edema, and inflammation. Our results have supported that the essential injury in APAP toxicity is tubular necrosis. In addition, NAC and NAC + OT prevented APAP-induced toxicity in the renal tissues. Kandis et al. have shown that NAC improve tubular injury in the APAP-induced nephrotoxicity.Citation33 Tubular changes were also significantly less in the NAC + OT rats.
In conclusion, the physiopathologic mechanisms in APAP-induced nephrotoxicity are complex and may include oxidative stress and inflammation. The findings of our study have shown that the combination of NAC and OT might protect the kidney from APAP-induced nephrotoxicity in a rat model. This combination might improve renal damages owing to both oxidative stress and inflammation.
ACKNOWLEDGMENTS
The authors express their sincere appreciation to FAVOR (FMF Arthritis Vasculitis and Orphan Diseases Research/www.favor.org.tr) web registries at Gulhane Military Medical Academy, Institute of Health Sciences, for their supports in epidemiological and statistical advisory and invaluable guidance for the preparation of the manuscript.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Yaman H, Isbilir S, Cakir E, Uysal B. Current issues with paracetamol induced toxicity. J Exp Integr Med. 2011;1:165–166.
- Inkielewicz-Stepniak I, Knap N. Effect of exposure to fluoride and acetaminophen on oxidative/nitrosative status of liver and kidney in male and female rats. Pharmacol Rep. 2012;64:902–911.
- Hanly LN, Chen N, Aleksa K, N-acetylcysteine as a novel prophylactic treatment for ifosfamide-induced nephrotoxicity in children: translational pharmacokinetics. J Clin Pharmacol. 2012;52:55–64.
- Ahmad ST, Arjumand W, Nafees S, Hesperidin alleviates acetaminophen induced toxicity in Wistar rats by abrogation of oxidative stress, apoptosis and inflammation. Toxicol Lett. 2012;208:149–161.
- Altinel O, Demirbas S, Cakir E, Comparison of hyperbaric oxygen and medical ozone therapies in a rat model of experimental distal colitis. Scand J Clin Lab Invest. 2011;71:185–192.
- Cakir E, Akgul OE, Aydin I, The association between neopterin and acetaminophen-induced nephrotoxicity. Ren Fail. 2010;32:740–746.
- Demirbas S, Cakir E, Akgul EO, Elevated serum neopterin levels in acetaminophen-induced liver injury. Environ Toxicol Pharmacol. 2011;31:165–170.
- Minami A, Tsuboi I, Harada T, Inflammatory biomarker, neopterin, suppresses B lymphopoiesis for possible facilitation of granulocyte responses, which is severely altered in age-related stromal-cell-impaired mice, SCI/SAM. Exp Biol Med (Maywood). 2007;232:134–145.
- Gul H, Uysal B, Cakir E, The protective effects of ozone therapy in a rat model of acetaminophen-induced liver injury. Environ Toxicol Pharmacol. 2012;34:81–86.
- Abraham P, Kanakasabapathy I, Dian BJ. Propylthiouracil attenuates acetaminophen-induced renal damage in the rat. Nephrology (Carlton). 2005;10:588–593.
- Ilbey YO, Ozbek E, Cekmen M, Melatonin prevents acetaminophen-induced nephrotoxicity in rats. Int Urol Nephrol. 2009;41:695–702.
- Bocci V, Zanardi I, Travagli V. Has oxygen-ozone therapy a future in medicine? J Exp Integr Med. 2011;1:5–11.
- Demirbag S, Uysal B, Guven A, Effects of medical ozone therapy on acetaminophen-induced nephrotoxicity in rats. Ren Fail. 2010;32:493–497.
- Bocci V. Is it true that ozone is always toxic? The end of a dogma. Toxicol Appl Pharmacol. 2006;216:493–504.
- Paulesu L, Luzzi E, Bocci V. Studies on the biological effects of ozone: 2. Induction of tumor necrosis factor (TNF-alpha) on human leucocytes. Lymphokine Cytokine Res. 1991;10:409–412.
- Bocci V, Paulesu L. Studies on the biological effects of ozone 1. Induction of interferon gamma on human leucocytes. Hematologica. 1990;75:510–515.
- Lowry OH, Rosebrough NJ, Farr AL, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169.
- Al-Fawaeir S, Akgul EO, Cayci T, Comparison of two methods for malondialdehyde measurement. J Clin Anal Med. 2011;2:11–14.
- Agilli M, Yaman H, Cayci T, Comparison of two different HPLC methods and Elisa method for measurement of serum neopterin. J Investig Biochem. 2012;1:43–47.
- Das J, Ghosh J, Manna P, Kumar BS. Taurine protects acetaminophen-induced oxidative damage in mice kidney through APAP urinary excretion and CYP2E1 inactivation. Toxicology. 2010;269:24–34.
- Palani S, Raja S, Naresh R, Sil PC. Evaluation of nephroprotective, diuretic, and antioxidant activities of plectranthus amboinicus on acetaminophen-induced nephrotoxic rats. Toxicol Mech Methods. 2010;20:213–221.
- Abdul Hamid Z, Budin SB, Wen Jie N, Hamid A, Husain K, Mohamed J. Nephroprotective effects of Zingiber zerumbet Smith ethyl acetate extract against paracetamol-induced nephrotoxicity and oxidative stress in rats. J Zhejiang Univ Sci B. 2012;13:176–185.
- Aydinoz S, Uzun G, Cermik H, Effects of different doses of hyperbaric oxygen on cisplatin-induced nephrotoxicity. Ren Fail. 2007;29:257–263.
- Chen N, Aleksa K, Woodland C, Rieder M, Koren G. N-Acetylcysteine prevents ifosfamide-induced nephrotoxicity in rats. Br J Pharmacol. 2008;153:1364–1372.
- Ueki M, Ueno M, Morishita J, Maekawa N. Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. J Biosci Bioeng. December 12, 2012. doi:pii:S1389-1723(12)00458-6.
- Zhao YL, Zhou GD, Yang HB, Rhein protects against acetaminophen-induced hepatic and renal toxicity. Food Chem Toxicol. 2011;49:1705–1710.
- Li C, Liu J, Saavedra JE, Keefer LK, Waalkes MP. The nitric oxide donor, V-PYRRO/NO, protects against acetaminophen-induced nephrotoxicity in mice. Toxicology. 2003;189:173–180.
- Cekmen M, Ilbey YO, Ozbek E, Simsek A, Somay A, Ersoz C. Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food Chem Toxicol. 2009;47:1480–1484.
- Sohn SH, Lee EY, Lee JH, Screening of herbal medicines for recovery of acetaminophen-induced nephrotoxicity. Environ Toxicol Pharmacol. 2009;27:225–230.
- Boutis K, Shannon M. Nephrotoxicity after acute severe acetaminophen poisoning in adolescents. J Toxicol Clin Toxicol. 2001;39:441–445.
- Sener G, Sehirli AO, Ayanoglu-Dulger G. Protective effects of melatonin, vitamin E and N-acetylcysteine against acetaminophen toxicity in mice: a comparative study. J Pineal Res. 2003;35:61–68.
- Kandis H, Erkan ME, Yildirim U, Comparison of the effects of N-acetyl cysteine and erdosteine in rats with renal injury caused by paracetamol intoxication. Hum Exp Toxicol. 2011;30:1350–1358.
- Bocci VA. Scientific and medical aspects of ozone therapy. State of the art. Arch Med Res. 2006;37:425–435.
- Caliskan B, Guven A, Ozler M, Ozone therapy prevents renal inflammation and fibrosis in a rat model of acute pyelonephritis. Scand J Clin Lab Invest. 2011;71:473–480.
- Oter S, Korkmaz A. Relevance of hyperbaric oxygen to ozone therapy. Arch Med Res. 2006;37:917–918; author reply 919.
- Oztosun M, Akgul EO, Cakir E, The effects of medical ozone therapy on renal ischemia/reperfusion injury. Ren Fail. 2012;34:921–925.
- Fouad AA, Yacoubi MT, El-Bidawy MH. Therapeutic potential of hemin in acetaminophen nephrotoxicity in rats. Environ Toxicol Pharmacol. 2009;27:277–282.
- Ruokonen E, Ilkka L, Niskanen M, Procalcitonin and neopterin as indicators of infection in critically ill patients. Acta Anaesthesiol Scand. 2002;46:398–404.
- Abdel-Zaher AO, Abdel-Hady RH, Mahmoud MM, Farrag MM. The potential protective role of alpha-lipoic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2008;243:261–270.
- Abraham P. Vitamin C may be beneficial in the prevention of paracetamol-induced renal damage. Clin Exp Nephrol. 2005;9:24–30.
