Abstract
Renal osteodystrophy is a common problem in renal failure patients. Bone pain is a common manifestation of renal osteodystrophy. The aim of the study was to assess the intensity of chronic bone pain via visual analog scale (VAS) and its relationship with parathyroid hormone, health-related quality of life (HRQoL), and depression in hemodialysis patients. Ninety-five patients recruited were asked to rate chronic bone pain via VAS. Depressive symptoms and HRQoL were assessed by Beck Depression Inventory (BDI) and Short-Form 36, respectively. VAS was positively correlated with intact parathyroid hormone (r = +0.322, p = 0.001), phosphorus (r = +0.300, p = 0.003), alkaline phosphatase (r = +0.275, p = 0.009), and negatively correlated with physical component (r = –0.320, p = 0.002) and mental component summary scores (r = –0.247, p = 0.016). In multivariate linear regression analysis, logVAS was independently associated with serum phosphorus (β = 0.072, 95% confidence interval: 0.020–0.123, p = 0.007), log intact parathyroid hormone (β = 0.176, 95% confidence interval: 0.041–0.310, p = 0.011), and physical component summary score (β = –0.018, 95% confidence interval: –0.031–(–0.005), p = 0.008). VAS is correlated with bone metabolism markers, namely, intact parathyroid hormone, and may be used to assess the intensity of chronic bone pain. The intensity of chronic bone pain is related with HRQoL in hemodialysis patients.
INTRODUCTION
The kidney plays a leading role in maintaining calcium-phosphorus homeostasis in collaboration with other organs, that is, the parathyroid gland, intestines, and bones. It is the target organ for various hormones, such as parathyroid hormone. Thus, along with the progression of chronic kidney disease, various abnormalities of mineral and bone metabolism develop, which cause morbidity and mortality.Citation1 Renal osteodystrophy is a painful syndrome unique to end-stage renal disease, which may develop during a patient’s time on dialysis. The three major types of renal osteodystrophy are osteitis fibrosa, osteomalacia, and adynamic bone disease, all of which are associated with bone pain.Citation2 Calcium–phosphorus homeostasis and parathyroid hormone are pivotal in bone metabolism and may be related with musculoskeletal pain in end-stage renal disease patients.Citation3
For the last few decades, dialysis has proved to be a successful life-sustaining therapy. The main outcome measure of dialysis effectiveness has been the patient survival.3 However, as the patients’ survival improves, health-related quality of life (HRQoL) becomes a more important focus. During chronic kidney disease, various abnormalities of mineral and bone metabolism develop, which cause decreased HRQoL.1
A growing body of literature has demonstrated that pain is one of the most common symptoms for patients with end-stage renal disease, impacting virtually every aspect of HRQoL.2 Chronic pain, of which the musculoskeletal pain is the most commonly encountered, is considered to be a highly relevant patient outcome in evaluating HRQoL in hemodialysis (HD).3 In fact, patients with end-stage renal disease are among the most symptomatic of any chronic disease group. Their chronic pain is typically moderate to severe in severity and is undertreated. There is a lack of recognition by the nephrology community concerning the extent and severity of the problem and hence a lack of clinical and research focus in this area.2
Depression not only influences the manifestations of chronic pain but complicates the treatment and interferes with the patient’s ability to cope with chronic pain. The role of depression in chronic pain in end-stage renal disease has been greatly under appreciated.2
By the light of these findings, we hypothesized that chronic bone pain, as assessed via visual analog scale (VAS), might be related with bone metabolism markers, HRQoL, and depression in end-stage renal disease patients in HD.
MATERIALS AND METHODS
The study had a cross-sectional design and was carried out in the HD unit in a secondary care state hospital in Turkey. The study conformed to the provisions of the Declaration of Helsinki in 1995 (as revised in Edinburgh 2000). Self-reported sociodemographic characteristics of patients included age, gender, previous renal transplantation (present/absent), presence of hypertension (present/absent), presence of diabetes mellitus (yes or no), presence of coronary artery disease (yes or no), presence of cerebrovascular disease (yes or no), and the presence of a sleep disturbance (yes or no). During the anamnesis procedure, we also recorded the highest educational level (illiterate, or elementary school, secondary school, high school, and university graduate), marital status (married, unmarried), economical status (monthly money income is satisfactory or not), and living arrangements (living with someone vs. living alone). The patients receiving erythropoietin injection were determined. Body mass index was calculated as the ratio of weight in kilograms to height squared (in square meters).
The dialysis prescription in our study included 4–5 h of HD thrice weekly for all patients with hollow fiber dialyzer, with blood flow rates of 300–400 mL/min, using a standard bicarbonate dialysis solution. Fasting blood samples were obtained before beginning the HD session to determine laboratory parameters, including serum hemoglobin, glucose, albumin, high-sensitive C-reactive protein, predialysis calcium and phosphorus, alkaline phosphatase, ferritin, predialysis blood urea nitrogen, creatinine, and intact parathyroid hormone (iPTH). Postdialysis serum urea nitrogen levels, used to calculate the urea reduction ratio, were measured. Urea kinetic modeling was performed in order to assess the delivered equilibrated dose of dialysis. HD dose was evaluated using the following formula:
where spKt/V is a single-pool Kt/V, R is the ratio of postdialysis to predialysis serum urea nitrogen, t is time on dialysis in hours, UF is the amount of ultrafiltration in liters, and W is postdialysis body weight in kilograms.
None of the patients had osteomyelitis and discitis complicating central lines/arteriovenous fistulas that can lead to painful ischemic neuropathies. Patients with recurrent pain due to needling, muscle cramps, and headaches during the HD procedure were excluded since these conditions may be perceived as chronic pain by some patients. In addition, patients with intermittent claudication, ischemic leg ulcer, and peripheral revascularization for amputation for critical limb ischemia within the last 3 months were excluded. None of the patients had cancer within the previous 2 years.
Assessment of Pain
The patients were asked to complete a pain survey to assess the intensity of chronic bone pain (lasting >3 months). The scale was continuous, horizontal, VAS, (0–100) with the anchor points, “no pain” and “worst imaginable pain,” respectively.Citation4
Beck Depression Inventory
To measure depressive symptoms, we used the original Beck Depression Inventory (BDI) form, as introduced by Beck et al. in 1961.Citation5 BDI is a 21-item self-reported inventory that measures characteristic attitudes and symptoms of depression. The 21 items are answered on a four-point Likert scale, where 0 represents the absence of a problem and 3 represents extreme severity of a problem. The total score ranges from 0 to 63. BDI was documented as a valid index of depression, and BDI scores correlate well with the diagnostic criteria for depression. The advantage of BDI involves its placement of a subject within a range of depression severity, rather than merely identifying whether the person meets certain diagnostic criteria. It is frequently used to measure depression in HD patients and is a useful screen for managing clinical depression in this patient group.Citation6 None of the patients were suffering from a major depressive episode during the study.
Health-Related Quality of Life
To evaluate the HRQoL of patients, we used Medical Outcomes Study Short-Form 36 (SF-36), which was adapted to the Turkish population.Citation7,Citation8 The test consists of 36 items and 8 subscales, that is, physical functioning, physical role (e.g., role limitations caused by physical problems), pain, general health, vitality, social functioning, emotional role (e.g., role limitations caused by emotional problems), and mental health. Each subscale was scored in a range of 0 to 100; the higher the score, the better the HRQoL. The pain subscale is scored so that a high score indicates freedom from pain. These eight subscales can be summarized according to two primary dimensions of functioning, that is, a physical component summary score and a mental component summary score. SF-36 is commonly used and is validated in patients with end-stage renal disease.Citation9
the patients received a brief explanation, assessment of depressive symptoms using BDI and assessment of HRQoL using SF-36 were performed for each patient during the HD session. The illiterate patients were assisted by the same HD practitioner during the assessment.
Biochemical Testing
Blood was obtained by venipuncture after an overnight fast. Complete blood counts were made by using the Beckman Coulter (Fullerton, CA, USA) automated blood counting device. Serum albumin was measured by bromcresol green method. High-sensitive C-reactive protein was measured using the CRP latex (II) immunoturbidimetric assay (Architech C 8000; Abbott Diagnostics, Abbott Laboratories, Abbott Park, IL, USA). Serum iPTH was measured by immunoassay method (Roche Diagnostics GmbH, Mannheim, Germany). Other biochemical tests were measured by standard laboratory methods.
Statistics
Statistical analysis was performed with SPSS software (Statistical Package for the Social Sciences, version 15.0; SPSS, Chicago, IL, USA). The results were considered statistically significant if the two-tailed p-value was <0.05. Normality of the data was evaluated by the Kolmogorov–Smirnov test. Data are shown as mean ± standard deviation, median [range (minimum–maximum)], or as a percentage (%), where appropriate. We used Spearman analysis for the correlation between VAS and other variables. Multivariate linear regression analysis was performed with enter method to determine the possible factors, [including gender, HD vintage, serum phosphorus, calcium, alkaline phosphatase, logiPTH, physical and mental component summary scores, and erythropoietin injection (yes/no), which has a common aftereffect of bone pain] independently related to VAS. Because VAS was not normally distributed, logarithmic conversion was performed before linear regression and scatter plot analyses.
RESULTS
In total, 95 HD patients were recruited. The etiology for end-stage renal disease was as follows: diabetes mellitus in 29 patients, hypertension in 23 patients, vesicourethral reflux and pyelonephritis in 5 patients, amyloidosis in 3 patients, glomerulonephritis in 8 patients, nephrolithiasis in 2 patients, polycystic kidney disease in 3 patients, ischemic nephropathy in 1 patient, and analgesic nephropathy in 1 patient. The etiology was unknown in 20 patients. demonstrates the demographic characteristics and erythropoietin usage in HD patients recruited in the study. demonstrates the laboratory characteristics. In , the BDI, SF-36, and VAS scores are shown.
Table 1. Demographic characteristics and erythropoietin usage in hemodialysis patients recruited in the study.
Table 2. Laboratory characteristics and spKt/V of hemodialysis patients recruited in the study.
Table 3. The Beck Depression Inventory, SF-36, and VAS scores of hemodialysis patients.
VAS was positively correlated with iPTH (r = +0.322, p = 0.001), phosphorus (r = +0.300, p = 0.003), and alkaline phosphatase (r = +0.275, p = 0.009). The regression graphics demonstrating the negative correlation between logVAS score and logarithmic transformed physical component summary and mental component summary scores of SF-36 are shown in and , respectively. The correlation of VAS score with BDI score and SF-36 subscales is shown in . In multivariate linear regression analysis, logVAS was independently associated with serum phosphorus, iPTH, and physical component summary score ().
Figure 1. Regression graphic demonstrating the negative correlation between logarithmic transformed VAS score and logarithmic transformed physical component summary score.
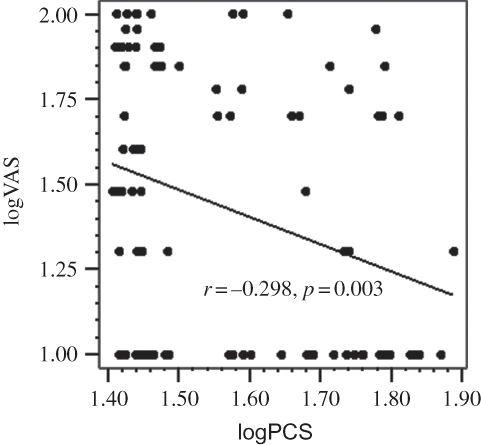
Figure 2. Regression graphic demonstrating the negative correlation between logarithmic transformed VAS score and logarithmic transformed mental component summary score.Notes: (logVAS, logarithmic transformed VAS score; logMCS, logarithmic transformed mental component summary score)
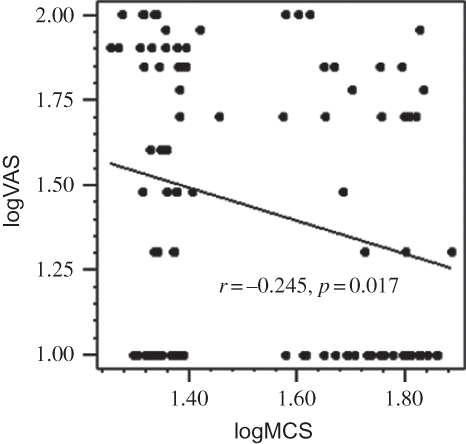
Table 4. The correlation of VAS scores of hemodialysis patients with Beck Depression Inventory score and SF-36 subscales.
Table 5. Multivariate linear regression analysis of potential predictors of logVAS in hemodialysis patients.
DISCUSSION
Pain is often described as the fifth vital sign, and health-care professionals are encouraged to regularly document the patient’s pain alongside other regular measurements that chart the patient’s course.Citation10 Chronic pain has a major impact on physical, emotional, and cognitive function, on social and family life, and on the ability to work and secure an income.4 Recent studies have shown that 37–50% of HD patients experience chronic pain, of which 82% is moderate to severe in intensity. The etiology of pain may be from numerous causes and is often multifactorial. Musculoskeletal pain from arthritis in elderly end-stage renal disease patients is one of the most common causes of chronic pain in this patient population.2 In our study, we asked the HD patients to rate their chronic bone pain and found that moderate to severe chronic bone pain was present in 49 (51.6%) (data not shown) HD patients, which is lower than the literature.
Renal osteodystrophy is a painful syndrome unique to end-stage renal disease that may develop during a patient’s time on dialysis. The three major types of renal osteodystrophy are osteitis fibrosa, osteomalacia, and adynamic bone disease, all of which are associated with bone pain.2 In our study, we found that the intensity of chronic bone pain, as assessed via VAS, was associated with bone metabolism markers, namely, serum iPTH, phosphorus, and alkaline phosphatase. Although we did not perform bone biopsy and histologically specify the type of renal osteodystrophy, our results showed a relationship between the intensity of chronic bone pain and routinely used biochemical bone metabolism markers, which is a practical issue in daily practice.
Pain is a multidimensional phenomenon with physical, psychological, and social components and is associated with psychological distress, impairment of interpersonal relationships, significant activity limitations in work, family, and social life. Therefore, not surprisingly, recent research in end-stage renal disease suggested that chronic pain is highly predictive of all domains of HRQoL.2 In our study, we demonstrated that chronic bone pain assessed via VAS was negatively correlated with all of the eight subscales of SF-36 (). We also found a negative correlation between the physical component summary score of SF-36 and VAS, and a negative correlation between the mental component score of SF-36 and VAS in the HD patient population. These results are in accordance with the literature.
Depression is common in end-stage renal disease patients with a prevalence of 5% to 50%, depending on the criteria used for diagnosis.2 In a prospective cohort study, Yamamoto et al. reported that depressive symptoms at baseline were significantly associated with higher odds of developing severe bodily pain during a 0.5- to 2.5-year follow-up period.Citation11 We did not find any significant correlation between the BDI score and chronic bone pain as assessed via VAS in correlation analysis. The discrepancy of our results from that of Yamamoto et al. may be due to the fact that our study was cross-sectional and that we only questioned for chronic bone pain, not the bodily pain, which has a broader definition.
Recent studies have failed to show an association between objective clinical assessments such as dialysis adequacy, serum albumin, hemoglobin, or calcium and phosphorous parameters with chronic pain, symptom burden, or HRQoL.2 We found a significant association between the intensity of chronic bone pain and serum iPTH and phosphorus. Golan et al. have reported that HD patients with chronic pain had statistically significant higher iPTH compared with those without chronic pain. However, they did not find any significant difference between the serum phosphorus levels of HD patients with and without chronic pain.3 This difference may result from the fact that they have investigated pain experienced in diverse organ systems (such as musculoskeletal pain, neuropathic pain, and headache), whereas we have investigated chronic bone pain.
One of the interesting findings in our study was that the VAS score was barely correlated with bodily pain subscale of SF-36. We think that this discrepancy is inherent in the definition of bodily pain subscale of SF-36, which may not solely include bone pain, but also refer to pain in a comprehensive manner such as headache, myalgia, and stomachache. In HD patients, different patterns of pain may coexist. Since we specifically referred to chronic bone pain in our pain assessment, our results may not be that much surprising in this manner.
We think that our results are promising in an attempt to improve clinical care of end-stage renal disease patients receiving HD. With timely interventions in calcium–phosphorus homeostasis and parathyroid hormone axis, there is little reason to not to believe that the chronic bone pain and therefore the HRQoL will improve. We think that future interventional studies are needed to clarify this issue. Of note, the VAS scores were lowest for the patients with polycystic kidney disease. The scores tended to be highest in diabetic patients in our cohort (data not shown). However, our study population was not powered enough to make a subgroup analysis. Therefore, we think that this issue may be another future research topic.
Our study has limitations that deserve mention. Firstly, and most importantly, we did not have the opportunity to sample bone tissue and histologically demonstrate the type or renal osteodystrophy in our patients. Addition of histological data would have improved our results significantly. Secondly, we were not able to measure more specific bone markers such as bone-specific alkaline phosphatase. Thirdly, we were not able to rule out other reasons of chronic pain in end-stage renal disease patients such as calciphylaxis, osteoarthritis, and dialysis-related amyloidosis. Fourthly, we did not measure serum vitamin D levels; therefore, we were not able to rule out pure osteomalacia. Lastly, assistance was available by the same HD practitioner for patients who were illiterate. Since the assistance was provided by the same caregiver of HD, who was not blinded to the patients’ status, this might have introduced some degree of bias.
In conclusion, our results demonstrate that VAS is related with bone metabolism markers, namely, iPTH, and may be used to assess the intensity of chronic bone pain in HD patients. The intensity of chronic bone pain, as determined via VAS, is related with HRQoL in end-stage renal disease patients in HD.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Komaba H, Tanaka M, Fukagawa M. Treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Intern Med. 2008;47:989–994.
- Davison SN. The prevalence and management of chronic pain in end-stage renal disease. J Palliat Med. 2007;10:1277–1287.
- Golan E, Haggiag I, Os P, Bernheim J. Calcium, parathyroid hormone, and vitamin D: major determinants of chronic pain in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1374–1380.
- Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101:17–24.
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571.
- Koo JR, Yoon JW, Kim SG, et al. Association of depression with malnutrition in chronic hemodialysis patients. Am J Kidney Dis. 2003;41:1037–1042.
- Ware JE Jr, Sherburne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483.
- Koçyigit H, Aydemir Ö, Fişek G, ÖLmez N, Memiş A. Kısa form-36 (kf-36)’ nın Türkçe versiyonunun güvenilirliği ve geçerliliği. İlac ve Tedavi Dergisi. 1999;12:102–106.
- Johansen KL, Painter P, Kent-Braun JA, et al. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int. 2001;59:1121–1127.
- Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798–804.
- Yamamoto Y, Hayashino Y, Akiba T, et al. Depressive symptoms predict the subsequent risk of bodily pain in dialysis patients: Japan Dialysis Outcomes and Practice Patterns Study. Pain Med. 2009;10:883–889.
