Abstract
Patients with small vessel vasculitis present fluctuating antineutrophil cytoplasmic antibodies (ANCA) levels to the point that positive ANCA may be missed even if only up to 10% of patients with microscopic polyangiitis (MPA) are ANCA-negative. The first-line treatment of MPA is the association of steroids and cyclophosphamide, especially in the presence of a rapidly progressive glomerulonephritis. Plasmapheresis, intravenous immunoglobulins, and tumor necrosis factor inhibitors have been proposed as alternative to standard therapy. Disseminated intravascular coagulation (DIC) is a possible event in the course of small vessel vasculitis. Gabexate mesylate is a protease inhibitor able to suppress endothelial cell injury, and it may be administered to treat DIC related to different diseases. In ANCA-associated vasculitis, cytokines play a key role in promoting endothelial damage. DIC-related thrombocytopenia may be misinterpreted as drug-induced because of the immunosuppressive properties of cyclophosphamide. Two cases of ANCA-positive MPA associated with DIC and treated with gabexate are reported in the literature with improvement of both hematological disorder and renal function. Our patient presented a rapidly progressive glomerulonephritis, and the renal biopsy showed MPA, in the absence of ANCA. After two weeks of steroid treatment, our patient developed a DIC. This case represents the first report of ANCA-negative MPA managed with gabexate, which showed improvement of coagulation disorders and kidney function. In conclusion, the anti-inflammatory properties of gabexate could be helpful in MPA at increased bleeding risk when immunosuppressive treatment is contraindicated, even in ANCA-negative vasculitis.
BACKGROUND
Microscopic polyangiitis (MPA) is a small vessel vasculitis associated in up to 90% of patients with antineutrophil cytoplasmic antibodies (ANCA).
Pulmonary and renal complications such as pulmonary hemorrhage and rapidly progressive glomerulonephritis are the most dangerous features of MPA. The first-line treatment of MPA is a combination of steroids and cyclophosphamide, although side effects limit their use, especially in the elderly or in patients who should not be treated with immunosuppressive agents because of their comorbidities.
CASE REPORT
A 69-year-old man was admitted to our department with general fatigue, nonproductive cough, and slight fever. His physical examination revealed peripheral edema, purpura, and splenomegaly. His blood pressure was 90/60 mmHg, pulse rate was 78/min, and regular with body temperature of 37.3˚C. Blood gas analysis showed metabolic acidosis (pO2 86, pCO2 29, pH 7.31, bicarbonate 14.6, and SpO2 96.2%). His medical history included diagnosis of short bowel syndrome as a result of a car accident 16 years before, and the patient had been on parental nutrition since then. Laboratory tests showed renal failure (creatinine 7.1 mg/dL and urea 119 mg/dL), anemia (Hb 7.1 g/dL), and thrombocytopenia. The inflammatory markers, erythrocyte sedimentation rate (ESR), and C-reactive protein were increased with complement C3 reduction. Tests for antinuclear antibodies, extractable ANCA, and antiplatelet antibodies were negative. Extractable ANCA antibodies were measured twice resulting all the time negative. Proteinuria (1.2 g/24 h) was documented with 24-h urine collection in association with microhematuria. Urinary cytology was negative for neoplastic cells.
A chest X-ray demonstrated diffuse reticular opacities with bilateral limited consolidations. A computerized tomography of the chest confirmed the presence of bilateral multiple interstitial thickening areas, morphologically characterized as pseudonodular. In addition, a bilateral pleural effusion was observed.
Urine culture and hemoculture were negative as well as serum parameters for common viral infections. Hence, we excluded an underlying infectious disease.
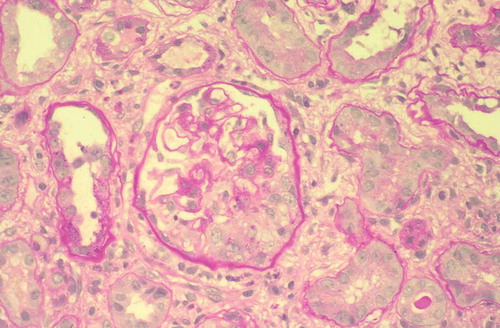
Because serum creatinine was 1.5 mg/dL 3 months before the admission, we suspected a rapidly progressive glomerulonephritis. Therefore, a renal biopsy was performed and the samples showed 21 glomeruli, 7 of which in global sclerosis, with 4 cellular crescents, 3 fibrocellular crescents and fibrinoid necrosis in the absence of deposits on immunofluorescence. Thus, a diagnosis of MPA was made ().
Figure 1. Proliferative extracapillary glomerulonephritis with the formation of a crescentic cell in the lower part of the glomerulus (PAS). × 100
A sudden worsening of pancytopenia contraindicated the association of steroids with cyclophosphamide, since it is already a side effect of drug.Citation1
Therefore, a steroid therapy was started (intravenous methylprednisolone at doses of 1 g/day for the first 3 days, followed by methylprednisolone at 60 mg/day).
During the second week of hospitalization, an impairment of renal function was observed (creatinine 9 mg/dL, urea 300 mg/dL, and GFR 6 mL/min), with diuresis reduction and worsening of metabolic acidosis. Thus, the patient was subjected to three hemodialysis sessions after which the renal function parameters improved (creatinine 4 mg/dL, urea 158 mg/dL, and GFR 16 mL/min) with diuresis recovery.
However, despite two weeks of steroid treatment, a diagnosis of disseminated intravascular coagulation (DIC) was hypothesized based on laboratory tests: fibrinogen 1.21 g/L (normal range 1.50–4.00), D-Dimer 1908 ng/mL (normal range 50–420), INR 1.6 (normal range 0.81–1.20), ratio 1.61 (normal range 0.80–1.30), platelets 45 × 109/L (normal range 150–450), and ATIII 65.5% (normal range 74–120) in association with gross hematuria.
The patient was also treated with blood transfusions, although useless and sometimes contraindicated. The decision to transfuse was based on symptoms (tachypnea, hypotension, fatigue, and dizziness) and hemoglobin concentration (7 g/dL). Nevertheless, pancytopenia with bleeding tendency and gross hematuria persisted.
Thus, we decided to treat the coagulation disorder by administering gabexate mesylate at doses of 1500 mg/day for 2 weeks. After two more weeks, laboratory tests documented a good response with improvement of coagulation test results (fibrinogen 3.46 g/L, D-Dimer 708 ng/mL, INR 1.22, ratio 1.22, PLT 99, and ATIII 75.51%) in the absence of gross hematuria and with stable creatinine levels.
After 6 months follow-up, laboratory findings showed a further improvement of both kidney function (serum creatinine 1.6 mg/dL, urea 76 mg/dL, and GFR 46 mL/min) and proteinuria (300 mg/24 h) ().
Table 1. Laboratory parameters in the course of gabexate administration.
DISCUSSION
The association of steroids and cyclophosphamide is the first-line therapeutic approach of MPA, especially in the presence of a rapidly progressive glomerulonephritis.Citation2
Almost all patients with small vessel vasculitis are ANCA-positive and only up to 10% of patients with MPA are ANCA-negative.Citation3
ANCA-negative patients are a minority of patients with MPA, and the pathogenesis of this subgroup of patients has not been completely investigated.
It is important to highlight that the ANCA levels can fluctuate between positive and negative, and thus periods of positive ANCA may be missed.Citation4
Our patient had a short bowel syndrome and for this reason had all the features of malnutrition, including leucopenia, whereby the use of cyclophosphamide was a contraindication itself.
In patients unresponsive to standard cytotoxic and immunosuppressive therapy, other options such as plasmapheresis, intravenous immunoglobulins, and tumor necrosis factor inhibitors have been employed in a small number of patients.Citation2
Our patient could not benefit from treatment with plasmapheresis because he was ANCA-negative. In the present case, gross hematuria gradually disappeared and an improvement of coagulation tests was observed in response to gabexate administration. Furthermore, after 2 weeks therapy with gabexate, serum creatinine levels remained stable in the absence of further dialysis treatment.
Gabexate mesylate inhibits proteases and suppresses endothelial cell injury and may be used to treat DIC ascribed to various kinds of diseases.Citation5,Citation6
Importantly, it is known that cytokines such as tumor necrosis factor-α (TNF-α) contribute to induce endothelial damage in ANCA-associated vasculitis, and gabexate could potently suppress TNF-α production and endothelial cell injury.Citation7,Citation8
The literature reported two cases of ANCA-positive MPA associated with DIC and treated with gabexate with improvement of hematological disorder and renal function.Citation9,Citation10
We assume that the improvement of our patient’s condition was due not only to the beneficial effect on coagulation disorders but inhibition of inflammatory pathways too.
In many cases of ANCA-associated vasculitis, the presence of latent DIC may be overlooked.
In addition, thrombocytopenia due to DIC may often be misinterpreted as a drug-induced disorder because of the immunosuppressive properties of cyclophosphamide.Citation9
Therefore, it may not be easy to detect the presence of latent DIC. In our patient, however, the immunosuppressive therapy for the treatment of MPA could not be used and we could diagnose DIC without any pharmacological interference.
This is the first case described in literature that reports the administration of gabexate in the course of ANCA-negative MPA. After 6 months, follow-up examination of our patient showed stable improvement of renal function in the absence of bleeding.
In conclusion, we suggest considering the use of gabexate in MPA patients who are, however, ANCA-negative and present a bleeding tendency.
Finally, as we demonstrated in our case, the association of gabexate mesylate with high-dose corticosteroids may be an effective therapy for some patients with MPA and DIC.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Goupil R, Brachemi S, Nadeau-Fredette AC, . Lymphopenia and treatment-related infectious complications in ANCA-associated vasculitis. Clin J Am Soc Nephrol. 2013;8(3):416–423.
- Mukhtyar C, Guillevin L, Cid MC, . European Vasculitis Study Group. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis. 2009;68(3):310–317.
- Eisenberger U, Fakhouri F, Vanhille P, . ANCA-negative pauci-immune renal vasculitis: histology and outcome. Nephrol Dial Transplant. 2005;20(7):1392–1399.
- Miller A, Chan M, Wiik A, Misbah SA, Luqmani RA. An approach to the diagnosis and management of systemic vasculitis. Clin Exp Immunol. 2010;160(2):143–160.
- Umeki S, Adachi M, Watanabe M, . Gabexate as a therapy for disseminated intravascular coagulation. Arch Intern Med. 1988;148:1409–1412.
- Gross WL, Schmitt WH, Csernok E. ANCA and associated diseases: immunodiagnostic and pathogenetic aspects. Clin Exp Immunol. 1993;91:1–12.
- Nakatani K, Takeshita S, Tsujimoto H, Kawamura Y, Sekine I. Inhibitory effect of serine protease inhibitors on neutrophil-mediated endothelial cell injury. J Leukoc Biol. 2001;69:241–247.
- Aosasa S, Ono S, Mochizuki H, Tsujimoto H, Ueno C, Matsumoto A. Mechanism of the inhibitory effect of protease inhibitor on tumor necrosis factor alpha production of monocyte. Shock. 2001;15:101–105.
- Saito T, Tsuchiya M, Shikata C, . Microscopic polyangiitis associated with marked systemic bleeding tendency caused by disseminated intravascular coagulation. Intern Med. 2003;42(9):850–855.
- Miyawaki K, Shiraishi J, Tsutsumi Y, . Beneficial effect of gabexate mesylate on microscopic polyangiitis with renal dysfunction and pulmonary hemorrhage: a case report. Angiology. 2006;57(4):522–525.
