Abstract
Tacrolimus, a calcineurin inhibitor, is a potent immunosuppressive agent used by a majority of transplanters across the globe. Its adverse effects include nephrotoxicity, neurotoxicity, new onset diabetes after transplant, gastro-intestinal toxicity, hepatotoxicity, and thrombotic microangiopathy. Tacrolimus-induced hepatotoxicity is a very uncommon side effect. We report a case of tacrolimus-induced hepatotoxicity in the form of cholestatic hepatitis a renal transplant recipient. Hepatotoxicity did not decrease after dose reduction; however, normalization of liver enzymes occurred after discontinuing tacrolimus.
INTRODUCTION
Tacrolimus-induced hepatotoxicity has been reported in lung and liver transplant recipients but is rare in renal transplant (RTx) recipients.Citation1–4 The incidence of cholestatic syndrome is 5.4% in pediatric liver transplant patients.Citation1 Tacrolimus-induced veno-occlusive of liver disease has been reported in RTx recipients.Citation4
CASE REPORT
A 28-year-old man weighing 50 kg with biopsy-proven benign nephrosclerosis underwent living-related RTx in our center in April 2012, with mother as donor. Before transplant, he was vaccinated with four doses of hepatitis B virus vaccine and was on maintenance hemodialysis for three months before RTx. During pretransplant evaluation, his liver function tests (LFT) were normal, ELISA for hepatitis B surface antigen (HBsAg) and hepatitis C virus (HCV) antibodies were nonreactive, and hepatitis B virus (HBV) DNA and HCV RNA were undetectable on PCR. Donor ELISA for HBsAg and HCV were also nonreactive. He did not receive any blood product during dialysis or transplant surgery.
After transplant, primary immunosuppression consisted of 500 mg methylprednisolone × 3 days followed by the maintenance of tacrolimus (0.08 mg/kgBW) keeping the target trough level between 4 and 7 ng/mL, mycophenolate sodium (1440 mg/day) and prednisolone, 20 mg/day. Prednisone was tapered to 10 mg/day by the end of 3 months. Fluconazole was given for 1 month; sulfamethoxazole-trimethoprim and ganciclovir were given as infection prophylaxis drugs for initial 3 months.
On 10th postoperative day, he had rise in serum creatinine to 1.8 mg/dL from nadir of 1.1 mg/dL. Renal allograft biopsy was performed and showed acute eosinophilic tubulointerstitial nephritis with acute B-cell-mediated rejection. He responded to antirejection therapy (methylprednisolone, 500 mg IV × 3, intravenous immunoglobulin, 5 g × 5 days, plasmapheresis × 4 sessions, and rituximab dose). After 10 days of treatment, SCr declined to 1.2 mg/dL.
He was admitted after 5 months of transplantation with complaints of anorexia, yellowish discoloration of eyes, and itching. On evaluation he was found to have altered LFT with serum bilirubin, 5.1 mg/dL (direct 4.1 mg/dL), aspartate aminotransferase (AST) 70 U/L (normal value 0–40 U/L), alanine aminotransferase (ALT) 137 U/L (normal value 0–40 U/L), alkaline phosphatase 1056 U/L (normal value 64–306 U/L), and glutamyl transferase 699 U/L (normal value 0–49 U/L). His viral markers, including ELISA against HCV and HBsAg, as well as HCV RNA, HBV DNA, and cytomegalovirus DNA by PCR and IgM against HEV and HAV, were negative. Antinuclear antibody, antidouble-stranded DNA antibody, antiliver–kidney–microsomal antibody, and antimitochondrial antibodies were found to be negative.
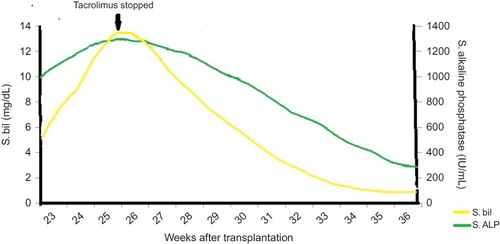
Abdominal ultrasound, Doppler for portal vein, and magnetic resonance cholangiopancreaticography (MRCP) pathology were unremarkable without any evidence of liver parenchymal lesion, biliary obstruction, or ascites. During this evaluation period of 1 week, his serum bilirubin gradually increased to 12.5 mg/dL (direct 9.6 mg/dL) with AST-99 IU, ALT-122 IU, and ALP-1278 IU. Coagulation profile was normal. The tacrolimus level was 6.1 ng/dL. Tacrolimus-induced hepatotoxicity was suspected since no other etiology was found despite extensive hepatic work-up. There is no history of intake of any over-the-counter or herbal preparations. Also, there was no other known offending drug causing cholestasis. The tacrolimus dose was therefore reduced to 0.05 mg/kgBW, and ursodeoxycholic acid was started to relieve intrahepatic cholestasis. However, serum bilirubin further increased to 13 mg/dL (direct bilirubin 11 mg/dL) over a period of 1 week. Hence, we withdrew tacrolimus and replaced it with everolimus. Liver biopsy was planned, but serum bilirubin started declining within 1 week of tacrolimus withdrawal and normalized after 8 weeks. ALP declined to normal after 10 weeks of stopping tacrolimus (). Renal allograft functions remained stable throughout the course of illness. Presently, after 8 months of transplantation, his LFT are normal and serum creatinine is 1.1 mg/dL on maintenance immunosuppression of prednisolone 10 mg/day, mycophenolate sodium 720 mg twice a day, and everolimus 1 mg daily.
DISCUSSION
Calcineurin inhibitors, including cyclosporin and tacrolimus, are the main components of immunosuppressant regimen in RTx. Hepatotoxicity has been reported with both drugs, but tacrolimus is much less common offender as compared to cyclosporine.Citation5 Cyclosporine is associated with risk of bile duct stones and sludge formation due to decrease in bile flow resulting from reduced bile acid secretion and is reported in 2–5% of transplant recipients.Citation6
Yaun et al. reported seven cases of cyclosporine-induced cholestatic hepatitis in RTx recipients and in all these cases switching to tacrolimus resulted in significant improvement in cholestatic papameters.Citation7
The exact mechanism of tacrolimus-induced hepatotoxicity is not known. In a rat model, it was found that high doses of tacrolimus induce cholestasis primarily by inhibiting biliary excretion of glutathione and bicarbonate without much effect on bile acid secretion.Citation8 Another mechanism of liver toxicity may be occurrence of veno-occlusive disease (VOD). There are reports of three cases in which cholestatic hepatitis was caused by tacrolimus by this mechanism and liver biopsy revealed VOD with a loose proliferation of subintimal reticulin beers extending into the vessel lumina, causing total or near total vascular occlusion of the terminal hepatic venules and sublobular veins. Serum bilirubin normalized within 2 months, but ALP normalized after 19–24 months of tacrolimus withdrawal.Citation4
In a retrospective analysis of 112 children who were switched to tacrolimus after orthotopic liver transplant, 6 of 112 (5.4%) developed cholestatic complications, mostly within 2 weeks after switching. In all patients with cholestasis, the trough levels of tacrolimus were <15 ng/L and bile duct system and the liver transplant were normal on ultrasound. Pruritus was present in all patients, and lab investigations showed elevated direct bilirubin without elevation of alkaline phosphatase. Liver biopsy showed hepatocellular necrosis and centrilobular cholestasis without signs of graft rejection. Switching back to cyclosporine and prednisolone in all six patients resulted in complete resolution of clinical signs and laboratory findings of cholestasis.Citation1
Taniai et al. reported a case of cholestatic jaundice due to tacrolimus after liver transplant. The patient improved completely after reducing the dose of tacrolimus and keeping the blood level of tacrolimus between 3 and 5 ng/dL.Citation2
Vikas et al. described a case of tacrolimus-induced hepatotoxicity developing 17 weeks after bilateral lung transplant. Abnormal liver function tests showed direct hyperbilirubinemia with marked rise in alkaline phosphatase. Tacrolimus dose reduction was done but without much improvement in liver enzymes. Change of therapy to cyclosporine caused further rise in liver enzymes. Cyclosporine was stopped, the patient was started on sirolimus and mycophenolate mofetil, and liver enzymes normalized 3 weeks after stopping cyclosporine.Citation3
We conclude that cholestatic jaundice in our patient was due to tacrolimus toxicity because even on extensive work-up we could not find out other etiologies of hepatic dysfunction and LFT completely normalized after tacrolimus was stopped.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ganschow R, Albani J, Grabhorn E, Richter A, Burdelski M. Tacrolimus-induced cholestatic syndrome following pediatric liver transplantation and steroid-resistant graft rejection. Pediatr Transplant. 2006;10(2):220–224.
- Taniai N, Akimaru K, Ishikawa YHepatotoxicity caused by both tacrolimus and cyclosporine after living donor liver transplantation. J Nippon Med Sch. 2008;75(3):187–191.
- Sacher VY, Bejarano PA, Pham SM. Tacrolimus induced hepatotoxicity in a patient with bilateral lung transplant. Transpl Int. 2012;25(10):e111–e1112. doi:10.1111/j.1432-2277.2012.01546.x
- Vallet-Pichard A, Rerolle JP, Fontaine HVeno-occlusive disease of the liver in renal transplant patients. Nephrol Dial Transplant. 2003;18(8):1663–1666.
- Soresi M, Sparacino V, Pisciotta GEffects of cyclosporin A on various indices of cholestasis in kidney transplant recipients. Minerva Urol Nefrol. 1995;47(2):65–69.
- Lorber MI, Van Buren CT, Flechner SM, Williams C, Kahan BD. Hepatobiliary complications of cyclosporine therapy following renal transplantation. Transplant Proc. 1987;19(1 Pt 2):1808–1810.
- Yuan QS, Zheng FL, Sun Y, Yu Y, Li Y. Rescue therapy with tacrolimus in renal graft patients with cyclosporine A-induced hepatotoxicity: a preliminary study. Transplant Proc. 2000;32(7):1694–1695.
- Sanchez-Campos S, Lopez-Acebo R, Gonzales P, Culebras JM, Tuñon MJ, Gonzalez-Gallego J. Cholestasis and alterations of glutathione metabolism induced by tacrolimus (FK506) in the rat. Transplantation. 1998;66(1):84–88.