Abstract
Non-Shiga-like toxin-producing Escherichia coli (STEC) or atypical hemolytic uremic syndrome (aHUS) is observed in 5–10% of all hemolytic uremic syndrome (HUS) cases, and usually develops secondary to infections, malignancies, drugs, transplantation, pregnancy, and autoimmune disease. However, there has been no report on adult onset HUS initiated by surgical procedures except transplantation. We report a 66-year-old woman who incurred renal impairment on the first day after laparoscopic hemicolectomy. Hemolytic anemia, thrombocytopenia, absence of Shiga toxin associated disease, normal ADAMTS13 activity, and low serum C3 (not C4) were consistent with a diagnosis of aHUS. We performed plasma exchange with fresh frozen plasma. Nevertheless, deteriorated renal function was not recovered after the treatment. Although it is an uncommon postoperative complication, aHUS needs to be considered as a possible cause of acute kidney injury combined with thrombocytopenia and anemia after surgical procedures, considering its different treatment modality and poor outcomes.
INTRODUCTION
Thrombotic microangiopathy (TMA) is a pathological process involving microvascular thrombosis, consumptive thrombocytopenia, and microangiopathic hemolytic anemia (MAHA) that leads to end-organ ischemia and infarction and most commonly affects the kidney and brain. The two main clinical syndromes comprising this common pathologic feature of TMA are thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). Although there is much overlap between the syndromes and some experts recommend treating them as a single entity,Citation1,Citation2 each has a distinct pathophysiological mechanism.Citation3 An imbalance between the levels of von Willebrand factor (vWF) and its cleaving protease (ADAMTS13) is the main mechanism involved in the pathophysiology of TTP.Citation4Citation,5 In contrast, HUS is characterized by the triad of hemolytic anemia, thrombocytopenia, and renal impairment and is usually preceded by an infection with Shiga-like toxin-producing Escherichia coli (STEC). Its toxin is a known culprit underlying endothelial cell damage and microthrombus formation within the kidneys.Citation6,Citation7 Non-STEC or atypical HUS (aHUS) is also observed in 5–10% of all HUS cases, and new insights into the role of the complement system in the pathogenesis of aHUS have emerged. Non-STEC–HUS usually develops secondary to infections, malignancies, drugs, bone marrow or solid organ transplantation, pregnancy, and autoimmune disease.Citation8 In particular, most cases of HUS secondary to surgical operation were posttransplant HUS as recurrent or de novo disease.Citation9 However, there have been no reports of HUS cases that developed secondarily to other surgical procedures in adult. We report a case of HUS with renal cortical necrosis following laparoscopic hemicolectomy.
CASE REPORT
A 66-year-old woman was admitted for recurrent constipation and abdominal pain. She had a history of hypertension although there was no specific family history. She was diagnosed with mechanical obstruction due to stenosis of the sigmoid colon and underwent laparoscopic left hemicolectomy. During the operation, her systolic blood pressure was constantly maintained higher than 100 mmHg and her blood loss was minimal (<50 mL). Histological examination of the specimen showed multiple ulcers with acute and chronic inflammation. Preoperatively, the patient had normal renal function (creatinine, 0.44 mg/dL), liver function (bilirubin, 0.30 mg/dL), and coagulation. She was anemic (hemoglobin 9.8 g/dL), but her platelet (242 × 103/μL) and white blood cell (7000/μL) counts were normal. One day after the operation, the patient’s urine output decreased and renal impairment was noted (creatinine 2.30 mg/dL). Her renal function deteriorated rapidly; 3 days postoperatively, pulmonary congestion developed and hemodialysis was initiated.
The patient’s platelet count and hemoglobin level decreased gradually, and 12 days postoperatively, her platelet count was 37 × 103/μL and hemoglobin level was 5.3 g/dL, necessitating transfusion of platelets and packed cells. Her serum lactate dehydrogenase (LDH) level was 1055 IU/L, plasma hemoglobin concentration was high, and haptoglobin level was low, all of which were consistent with hemolysis. A peripheral blood smear demonstrated fragmented red blood cells (schistocytes) and thrombocytopenia. The findings for Coombs test were negative. Fever or neurologic abnormality was not noted. On ultrasonography, increased renal cortical echogenicity was noted; however, there were no other pathological findings around the kidney.
Based on the findings of acute kidney injury (AKI), thrombocytopenia, and MAHA, HUS was diagnosed. Considering the pathogenetic mechanisms of TMA, a double-volume plasma exchange was performed using fresh frozen plasma.
Specific tests were conducted to delineate the nature of the TMA: Stool culture was negative for E. coli O157, and HIV serology was negative. Prothrombin time, activated partial thromboplastin time, and fibrinogen were within the reference ranges. C4 level was normal (17.7 mg/dL; reference range, 16–47 mg/dL), C3 level was low (62.3 mg/dL; reference range, 88–201 mg/dL), ADAMTS13 activity was normal (55%; reference range, 44–121%), and ADAMTS13 inhibitors were negative. The results for antinuclear antibodies (Abs) including anti-double-stranded DNA Abs, anti-Smith Abs, and anti-histone Abs as well as anti-phospholipids Abs, such as anticardiolipin Abs, anti-beta2 glycoprotein1 Abs, and lupus anticoagulant, were all negative.
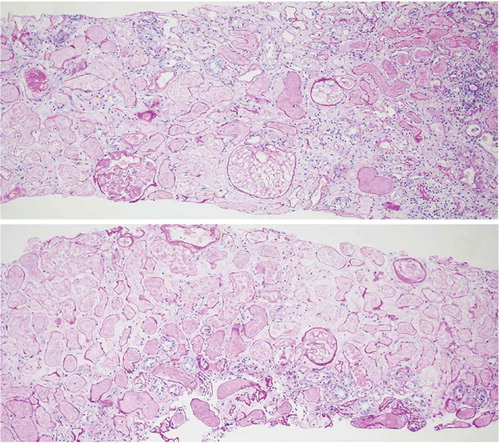
After three sessions of plasma exchange, the patient’s platelet count increased to 205,000 and LDH decreased to 395, and the hematologic remission was sustained. However, since she still required dialysis, a renal biopsy was performed 22 days after surgery. Histological findings showed complete necrosis of the renal cortex and medulla as well as fibrin thrombi in some glomeruli and interlobular vessels, all of which are consistent with TMA (). The patient was discharged but continued hemodialysis on an outpatient basis.
DISCUSSION
HUS and TTP have common pathologic features and clinical similarities. However, with the discovery of vWF-cleaving metalloprotease ADAMTS-13 and its role in the pathogenesis of TTP, they are recognized as clearly different disease entities.Citation3 In addition, among various secondary causes of HUS and TTP including infection, pregnancy, autoimmune disease, medication, and transplantation, surgical procedures except for transplantation are usually complicated by TTP rather than HUS. Since the first report of postoperative TTP by Hirsh in 1982,Citation10 several similar cases have been described, the majority of which resulted from cardiac or vascular surgeries.Citation11 For HUS, after the case of postoperative HUS in a child by Maki in 1968,Citation12 only de novo posttransplant HUS cases have been reported.Citation9 After a kidney transplant operation, the allograft tissue is placed in a situation that is more susceptible to endothelial damage by alloimmune responses, especially those mediated by antihuman leukocyte antigen antibodies, ischemia–reperfusion injuries, immunosuppressive drugs, and viral infections. Such combined factors may act synergistically in the development of HUS. However, other surgical procedures such as laparoscopic hemicolectomy, as in the present case, have not been reported as an initiating factor of HUS, especially in adults.
In this case, the patient had the typical triad of hemolytic anemia, thrombocytopenia, and renal impairment as well as fibrin thrombi in the interlobular vessels, all of which are consistent with HUS, although no typical triggering event such as infection (STEC or non-STEC), medication, systemic disease, or other malignancy was found. Interestingly, the patient had reduced serum levels of complement fraction C3 and normal levels of C4, suggesting activation of the complement system and complement consumption.
Complement dysregulation in the endothelium has been shown to play a critical role in the pathogenesis of aHUS. Experimental and clinical data have shown that aHUS is associated with a mutation in the gene of complement-inhibiting factors or complement-activating factors, which leads to unopposed activation of the complement system.Citation8 Such mutations in the genes for C3 convertase protein, C3, factor B, and complement regulatory proteins such as factor H, membrane cofactor protein, and factor I or thrombomodulin have been noted in aHUS cases.Citation8Citation,13 These mutations result in dysregulation of the complement system that leads to excessive complement activation and endothelial damage. Despite genetic disorders of the complement system, most cases of aHUS are sporadic, with familial cases comprising <20% of all cases.Citation8 This indicates that an environmental factor contributes to disease development. Triggers for the sporadic form include infection with the human immunodeficiency virus, malignancy, transplantation, pregnancy, and the use of certain anticancer drugs, immune suppressants (e.g., cyclosporine, tacrolimus), or antiplatelet agents (e.g., ticlopidine, clopidogrel).14–16 In the present case, the absence of STEC disease, normal ADAMTS13 activity, and low serum C3 (but not C4) level were consistent with aHUS. More importantly, laparoscopic hemicolectomy might be a triggering factor that leads to endothelial damage in conditions of complement dysregulation, although the genetic profile responsible for complement dysregulation was not assessed in this case.
Despite the decreased incidence of renal cortical necrosis and improved survival in TTP–HUS,Citation17Citation,18 the overall outcome of aHUS is poor compared with STEC–HUS. Up to 25% of the patients may not survive the acute phase, and up to 50% of these patients progress to end-stage renal disease (significantly worse in adults than in children).Citation8,Citation19 Therefore, urgent treatment before the confirmation of laboratory data should be recommended. Although there is no evidence from controlled trials for the treatment of HUS in adults according to the American Medical Association (AMA) indications,Citation20 some reports showed about 30–60% response rates of short-term plasma therapy in aHUS. Therefore, plasmapheresis can be considered as the first choice of treatment for aHUS.Citation8 We also performed a plasma exchange; however, the patient’s renal function did not recover after treatment. We also performed a kidney biopsy to collect etiological or prognostic information and found diffuse complete necrosis of the renal cortex and medulla as well as fibrin thrombi in the glomeruli and interlobular vessels—findings that are compatible with TMA.
AKI is a common complication after any operation and is associated with poor outcomes during hospitalization; early diagnosis and intervention are required to mitigate its negative effects. However, when AKI develops with thrombocytopenia and anemia, it is difficult to consider postoperative HUS as a diagnosis because no such case has been reported in adults and those abnormalities of laboratory data can easily occur after an operation due to other etiologies. Here, we reported the first case of postoperative HUS in an adult following laparoscopic hemicolectomy. Accordingly, clinicians need to have a high index of suspicion of HUS in such situations considering its different treatment modality and poor outcomes.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Remuzzi G. HUS and TTP: variable expression of a single entity. Kidney Int. 1987;32:292–308.
- Clark WF. Thrombotic microangiopathy: current knowledge and outcomes with plasma exchange. Semin Dial. 2012;25:214–219.
- Motto D. Endothelial cells and thrombotic microangiopathy. Semin Nephrol. 2012;32:208–214.
- Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347:589–600.
- Sadler JE, Moake JL, Miyata T, George JN. Recent advances in thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program. 2004:407–423.
- Noris M, Remuzzi G. Hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:1035–1050.
- Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and hemolytic uraemic syndrome. Lancet. 2005;365:1073–1086.
- Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687.
- Zuber J, Le Quintrec M, Sberro-Soussan R, Loirat C, Fremeaux-Bacchi V, Legendre C. New insights into postrenal transplant hemolytic uremic syndrome. Nat Rev Nephrol. 2011;7:23–35.
- Hirsh LF. Vasculitis, thrombotic thrombocytopenic purpura, and stroke after aneurysm surgery. Surg Neurol. 1982;17:426–428.
- Naqvi TA, Baumann MA, Chang JC. Post-operative thrombotic thrombocytopenic purpura: a review. Int J Clin Pract. 2004;58:169–172.
- Maki S, Miyata H, Uda H. Hemolytic-uremic syndrome: fatal case after operation for intussusception as complication of upper respiratory tract infection. Med J Osaka Univ. 1968;19:157–173.
- Taylor CM, Machin S, Wigmore SJ, Goodship TH. Clinical practice guidelines for the management of atypical hemolytic uraemic syndrome in the United Kingdom. Br J Hematol. 2010;148:37–47.
- Besbas N, Karpman D, Landau D, et al. A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int. 2006; 70:423–431.
- Ruggenenti P, Noris M, Remuzzi G. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int. 2001;60:831–846.
- Zakarija A, Bennett C. Drug-induced thrombotic microangiopathy. Semin Thromb Hemost. 2005;31:681–690.
- Prakash J, Vohra R, Wani IA, et al. Decreasing incidence of renal cortical necrosis in patients with acute renal failure in developing countries: a single-centre experience of 22 years from eastern India. Nephrol Dial Transplant. 2007;22:1213–1217.
- Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 1991;325:398–403.
- Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–1859.
- Kaplan AA. Therapeutic plasma exchange: core curriculum 2008. Am J Kidney Dis. 2008;52:1180–1196.