Abstract
Despite the fact that low plasma zinc (Zn) levels play important roles in the oxidative stress, the relationships between lipid peroxidation and inflammation biomarkers with low plasma Zn levels have not been investigated in chronic kidney disease (CKD) patients. The aim of this study was to evaluate the Zn plasma levels, electronegative LDL [LDL(–)] levels, and inflammation markers as predictors of cardiovascular (CV) mortality in hemodialysis (HD) patients. Forty-five HD patients (28 men, 54.2 ± 12.7 years, 62.2 ± 51.4 months on dialysis and BMI 24.3 ± 4.1 kg/m2) were studied and compared to 20 healthy individuals (9 men, 51.6 ± 15.6 years, BMI 25.2 ± 3.9 kg/m2) and followed for 24 months to investigate the risks for CV mortality. LDL(–) levels were measured by ELISA, plasma Zn levels by atomic absorption spectrophotometry, C-reactive protein (CRP) level by immunoturbidimetric method, and tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1), and plasminogen activator inhibitor-1 (PAI-1) levels by a multiplex assay kit. HD patients presented low plasma Zn levels (54.9 ± 16.1 μg/dL) and high-LDL(–) (0.18 ± 0.12 U/L) and TNF-α (5.5 ± 2.2 pg/mL) levels when compared to healthy subjects (78.8 ± 9.4μ g/dL, 0.10 ± 0.08U/L, 2.4 ± 1.1 pg/mL, respectively, p < 0.05). Zn plasma levels were negatively correlated to TNF-α (r = –0.49; p = 0.0001) and LDL(–) (r = –0.33; p = 0.008). During the 2 years, 24.4% of the patients died, all due to CV disease. Analysis by the Cox model showed that high CRP, TNF-α, IL-6 levels, and long duration of HD were significant predictors of mortality. In conclusion, reduced Zn levels were associated with lipid peroxidation and inflammation, and we confirm here in a Brazilian cohort of HD patients that inflammation markers are strong predictors of CV death.
INTRODUCTION
Oxidative stress is a condition characterized by excessive production of reactive oxygen species (ROS) which overwhelms the antioxidants defense particles, such as zinc (Zn). In patients with chronic kidney disease (CKD), the Zn deficiency is common as a result of uremia, reduced renal function, diet, medication, losses in dialysis treatment, and an impaired absorption of Zn through the intestine.Citation1–Citation5 Due to the biological importance of Zn as an antioxidant agent, its deficiency could contribute to the development of atherosclerotic cardiovascular (CV) disease in CKD patients.Citation6
In this sense, the deficiency of antioxidants in these patients has been associated with increased oxidative stress, and can be involved in the generation of a pro-apoptotic subfraction of LDL, called electronegative LDL [LDL(–)].Citation7,Citation8 LDL(–) is a plasma-circulating LDL subfraction, minimally modified, that can be an initiating factor of atherosclerosis by actively contributing to alteration of the vascular endothelium and induce the cell death, thus increasing CV risk.Citation8–Citation10 Many reports have described the relationship between an increase in LDL(–) levels and increased CV disease risk in patients with hypercholesterolemia and diabetes mellitus.Citation10–Citation12 Our group also found high-LDL(–) levels in patients undergoing hemodialysis (HD),Citation13 suggesting that this increased levels of LDL(–) could be involved in the progress of the atherogenic process in CKD patients.
Taking these observations into account, we could infer that Zn deficiency may play an important role in the generation of oxidative stress and may be associated with inflammation and increased levels of LDL(–) in CKD patients. In this context, the aim of the present study was to evaluate the association between Zn plasma levels, inflammation, and lipid peroxidation markers and the risk factors for CV mortality in Brazilian HD patients.
SUBJECTS AND METHODS
Forty-five HD patients (28 men, 54.2 ± 12.7 years, 62.2 ± 51.4 months on dialysis and BMI 24.3 ± 4.1 kg/m2) from RenalCor Clinic in Rio de Janeiro, Brazil, were studied and compared to 20 healthy individuals (9 men, 51.6 ± 15.6 years, BMI 25.2 ± 3.9 kg/m2). Inclusion criteria were age >18 years and patients on maintenance dialysis for at least 6 months. Patients with cancer, AIDS, auto-immune disease, and the previous use of catheter for dialysis access were excluded.
The dialysis procedure was 3–4.5 h/session three times per week, blood flow greater than 250 mL/min, dialysate flow 500 mL/min, 100 and bicarbonate buffer. Renal failure etiologies included systemic arterial hypertension (n = 23), diabetes (n = 9), chronic glomerulonephritis (n = 4), polycystic kidney disease (n = 2), and unknown (n = 7). The study protocol was approved by the Ethics Committee of Clementino Fraga Filho University Hospital (HUCCF-UFRJ).
METHODS
Nutritional Assessment
The following anthropometric parameters were measured: body weight, height, and waist circumference (WC). Body mass index (BMI) was calculated from the equation BMI = weight (kg)/height2 (m).14 Measurements were made after the dialysis session by a trained staff member.
BIOCHEMICAL ANALYSES
Blood samples were obtained from the arterial line of the HD before the start of the session, after the patients had fasted overnight. The serum was drawn into EDTA (ethylenediaminetetra-acetic acid) containing syringe (1.0 mg/mL), and the plasma was immediately separated and stored into tubes containing Butylated hydroxytoluene (BHT) (100 μM), aprotinin (10.0 μg/mL), benzamidine (10.0 μM) and phenylmethanesulfonylfluoride (PMSF) (5.0 μM), at –80°C, until inflammation markers and LDL(–) analysis. For Zn analysis, blood was collected in plastic, Zn-free tubes with sodium citrate at 30% as anticoagulant.
The total cholesterol (TC), HDL-cholesterol (HDL-c) and LDL-cholesterol (LDL-c) were calculated according to Friedewald’s formula. The following parameters were measured using the standard laboratory measurements: Triglycerides (TG), hemoglobin (Hb), hematocrit (Hct), and albumin. The levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), Plasminogen activator inhibitor-1(PAI-1), and Monocyte chemotactic protein-1(MCP-1) were measured with a multiplex assay kit manufactured by R&D Systems® (Minneapolis, MN, EUA) in a Luminex® 100/200™ System (Austin, TX, USA). The levels of C-reactive protein (CRP) were measured by immunoturbidimetric method, using kit BioSystem® (Costa Brava, Barcelona, Spain).
Determination of Plasma Zn Levels
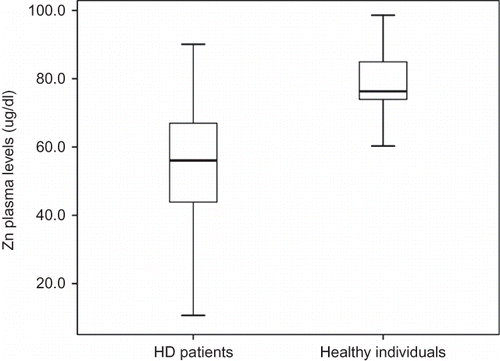
Plasma Zn was assayed by flame atomic absorption spectrophotometry (Varian AA240 Fast Sequential AAS Inc., Santa Clara, CA, USA), according to Rodriguez et al.Citation15 at the standard wavelength of 213.9 nm. A linear standard curve resulted, and a correlation coefficient (r) of 0.999 was calculated from it. The normal Zn concentration values are higher than 70 μg/dL in plasma (Gibson, 1990).Citation16
Electronegative LDL Isolation
LDL(–) was obtained as described in Faulin et al.Citation17 Briefly, the LDL fraction was separated by ultracentrifugation of human blood plasma from volunteers. LDL(−) was isolated from LDL by FPLC (BioLogic Duo Flow, Bio-Rad Laboratories Inc., Hercules, CA, USA) using an ion exchange column (Sepharose UNO Q-12, Bio Rad Laboratories Inc.) and eluted with a gradient constituted by 20 mM TRIS, pH 7.4 (pump A), and 20 mM TRIS + 1 M NaCl pH 7.4 (pump B). The eluent was monitored by UV at 280 nm and the LDL(–) collected peak was visualized by agarose gel electrophoresis. This purified LDL(–) subfraction was used as a standard in the ELISAs for LDL(–).
Detection of LDL(–) in Blood Plasma
Ninety-six-well flat-bottomed polystyrene plates (Costar, Corning Inc, NY, USA) were coated with 0.5 μg/well of anti-LDL(–) 1A3H2 monoclonal antibody in 0.05 M carbonate-bicarbonate buffer, pH 9.6, at 4°C overnight. The plates were washed three times with PBS, pH 7.4, containing 0.05% Tween 20. The plates were blocked by adding PBS containing 2% non-fat dry milk and 0.01% Tween 20 for 1.5 h at 37°C, then washed as described above. Plasma diluted in PBS containing 1% non-fat milk and 0.01% Tween 20 was added to the plates and incubated for 1.5 h at 37°C. The plates were washed and incubated with 0.5 μg/well of anti-LDL(–) 2C7D5F10 monoclonal antibody conjugated to biotin for 1 h at 37 °C. After washing, streptavidin conjugated horseradish peroxidase (Invitrogen Corp., Carlsbad, CA, USA) was added and incubated for 1h at 37°C. After washing, plates were incubated with ortho-phenylenediamine (OPD) diluted in citrate phosphate buffer, pH 5.3, at 37°C for 15 min. The reaction was stopped by adding 2 M sulfuric acid, and the absorbance at 492 nm was measured by spectrophotometry using a plate reader (SynergyTM Mx, Biotek Instruments Inc, Winooski, VT, USA).
Statistical Analysis
All data were analyzed for normality of distribution using the Kolgomorov–Sminorv test. The data are expressed as mean ± SD (Standard Deviation) or medians (min–max), as applicable. A p-value of <0.05 was considered to be statistically significant. Differences between the groups were analyzed by t-test or the non-parametric Mann–Whitney test as appropriate. The Spearman or Pearson correlation coefficients were used to determine the relationships between clinical parameters. The influence of Zn levels, inflammation, and oxidative stress markers over CV mortality was examined with a Cox model. Statistical analyses were performed using the SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA).
RESULTS
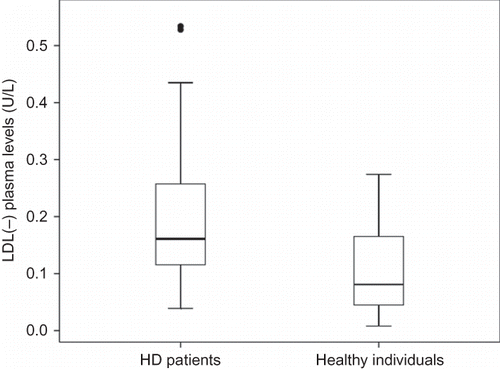
The demographic, clinical, and laboratory characteristics of the patients and healthy individuals are listed in . The plasma Zn levels and inflammatory markers of the patients and healthy individuals are listed in . Age, gender, BMI, and WC distribution in both the groups were similar. The patients presented significantly high levels of TNF-α, CRP, VLDL-c, and TG, and significantly decreased levels of total cholesterol, HDL, and LDL cholesterol when compared to those of healthy individuals. Plasma Zn levels were significantly lower in HD patients (54.9 ± 16.1 μg/dL) than in healthy individuals (78.8 ± 9.4 μg/dL) (p < 0.001) () and hypozincemia prevalence was 83.3% in the patients. LDL(–) levels were higher in HD patients (0.18 ± 0.12 U/L) than in healthy individuals (0.10 ± 0.08 U/L) (p < 0.04) (). There was no correlation between LDL(–) levels and inflammatory markers. The parameters analyzed were not different between diabetic and non-diabetic patients.
Table 1. General characteristics of the HD patients and healthy individuals.
Table 2. Plasma Zn levels and inflammatory markers in HD patients and healthy individuals.
Zn plasma levels were negatively correlated to TNF-α (r = –0.49; p = 0.0001) and LDL(–) (r = –0.33; p = 0.008). During the 2 years, 24.4% of the patients died, all due to CV disease. Analysis by the Cox model showed that high CRP (HR: 1.015, CI: 1.01–1.03, p < 0.001), TNF-α (HR: 1.33, CI: 1.04–1.75, p < 0.02), IL-6 levels (HR: 1.44, CI: 1.03–2.03, p < 0.03) and HD duration (HR: 0.88, CI: 0.83 – 0.96, p < 0.009) were significant predictors of mortality.
DISCUSSION
According to previous studies,1–5 our data also demonstrated Zn deficiency in HD patients. In addition, our study demonstrated for the first time, a correlation between this deficiency with high TNF-α and LDL(–) levels in HD patients, suggesting the crucial role of Zn as an antioxidant in these patients.
In fact, Zn is an essential mineral and there are many mechanisms by which Zn reduces the production of inflammatory cytokines and the oxidative stress. Zn plays a role of endothelial nitric oxide synthase, stabilizing the dimer of two subunits of this enzyme which is responsible for the production of nitric oxide (NO) that plays a substantial role in the function of platelets and leukocytes.Citation18 Studies have shown that Zn deficiency leads to changes in the production of NO, which is associated with the development of atherosclerosis.Citation19,Citation20 Zn also appears to be essential for the protective properties of PPARs (activated receptors of the peroxisome proliferator) which seems to play an anti-inflammatory role in the protection against activation of endothelial cells.Citation21,Citation22
Nevertheless, Zn plays an important role in the modulation of inflammatory/immune response, and a good availability of this trace element is associated with reduced plasma levels of IL-6 and TNF-α.Citation23 In addition, Prasad et al.Citation24 showed that the low availability of Zn promotes the development of atherosclerosis.
Zn also plays an important role in the activity of superoxide dismutase (SOD) which inhibits NADPH oxidase and, effectively decreases the hydroxyl radical, a ROS. The ROS activate NF-κB (Nuclear factor Kappa B), which induces the generation of pro-inflammatory cytokines and adhesion molecules.Citation24,Citation25
Shen et al.Citation26 observed that Zn deficiency increases oxidative stress and activity of NF-κB, induces cyclooxygenase-2 (COX-2), and expression of E-selectin, as well as the adhesion of monocytes to endothelial cell cultures, suggesting that Zn can modulate the mechanisms of inflammatory diseases such as atherosclerosis.
Another mechanism by which Zn reduces the production of inflammatory cytokines involves protein regulation by Zn (zinc finger protein), like protein A20, which inhibits TNF-α and IL-1β; thus, Zn acts as an antioxidant and anti-inflammatory.Citation27
Zn may play an antiatherogenic role through the inhibition of inflammation and oxidative stress, consequently inhibiting the oxidation of LDL particles by macrophages and endothelial cells.Citation20,Citation28–Citation30
LDL(–) (a lipid peroxidation marker) induces atherogenesis by a variety of biological activities including cytotoxicity and leukocyte recruitment that actively contribute to the alteration of the vascular endothelium, increasing CV risk.Citation8–Citation10 Many reports have described the increased LDL(–) levels in patients with CV disease risk, including CKD.Citation11–Citation13
The presence of electronegative LDL in the bloodstream stimulates several immune system components that are associated with atherosclerosis. These biomarkers include macrophages, T cells, interleukins (IL-1, IL-2, IL-6, IL-8, IL-10, and IL-12), TNF-α, gamma interferon, growth factors, platelet derived, MCP-1, and VCAM-1.Citation31
These studies suggest that the increased levels of LDL(–) can be involved in the progress of the atherogenic process, and that Zn could protect against this process. In fact, cellular enrichment with Zn has been shown to attenuate or prevent TNF-induced endothelial cell injury.Citation32
According to Meerarani et al.,Citation29 Zn deficiency increases apoptotic cell death induced by TNF-α, and these authors have showed that Zn supplementation attenuates apoptosis. Moreover, several potential mechanisms may account for the benefits of Zn, the increased activity and the expression of stress-related proteins and antioxidant proteins, including metallothioneins (MT), chaperones, ApoJ, Poly (ADP-Ribose) polymerase-1 (PARP-1) methionine sulfoxide reductase (Msr), and superoxide dismutase (SOD).Citation33–Citation36
Bao et al.Citation30 observed that 6 months of Zn supplementation to elderly subjects caused an increased Zn plasma level and reduced the high levels of inflammatory markers like the sensitivity of CRP, IL-6, MCP-1, VCAM-1, secretory phospholipase A2, and malondialdehyde and hydroxyalkenals (MDA + HAE). In addition, they showed in cell culture that Zn decreased the generation of TNF-α, IL-1b, VCAM-1, and MDA + HAE, and the activation of NF-κB and the increased anti-inflammatory proteins A20 and peroxisome proliferator activated receptor-α in human monocytic leukemia THP-1 cells and human aortic endothelial cells compared with Zn-deficient cells.
According to the studies cited above, our results suggest that Zn deficiency in HD patients might predispose to oxidative stress and inflammation. In the study presented here, only inflammation markers (high CRP, TNF-α, and IL-6 levels) and HD duration were predictors of CV mortality, which is in agreement with several other reports.Citation37–Citation39
CRP, IL-6, and TNF-α are potent pro-inflammatory cytokines and play a critical role in the development of several chronic inflammatory diseases, such as atherosclerosis.Citation40
Zoccali et al.Citation41 found that IL-6 predicts the inflammation charge in chronic renal patients, being the ideal indicator of the severity of inflammation and its use as a biomarker of mortality is recommended. Fellah et al.Citation42 and Kuragano et al.Citation43 also found that high levels of CRP and TNF-α were independently associated with CV mortality.
Furthermore, the rate of CV mortality in HD patients continues to increase and this is due to persistent exposure to CV risk factors inherent to the disease and the treatment of HD.Citation44–Citation46
It should be noted that our study presents some limitations; the most important is our inability to infer causality from the observed associations. This limitation is inherent in cross-sectional and observational studies and our study was also limited in that the number of patients included was relatively small.
Therefore, Zn appears to be crucial for protection against atherosclerosis. Indeed, we demonstrated for the first time that Zn plasma levels were negatively correlated with LDL(–) and TNF-α levels in HD patients. Our findings are very important because they provide further evidence that Zn deficiency could contribute to the development and progression of atherosclerosis.
In conclusion, reduced Zn levels were associated with increased LDL(–) and TNF-α levels in HD patients suggesting a relationship between Zn deficiency, lipid peroxidation and inflammation in these patients. It would be reasonable that frequent evaluation of Zn status and supplementation of Zn to this population at high risk of atherosclerosis should be considered as a strong candidate for further clinical and epidemiological investigations to better assess the risk of atherosclerosis that would help clinicians define/redefine their therapeutic approach. Moreover, based on these data, interventions are necessary to correct inflammation in HD patients due to the relationship with CV mortality.
Declaration of interest
There are no conflicts of interest to declare.
Dr. Torres and Dr. Mafra are researchers at CNPq and Jovem Cientista do Nosso Estado (FAPERJ), and Dr. Torres is Advance Selikoff Fellow of the Mount Sinai School of Medicine and is partially funded by Grant 1D43TW00640 from the National Institutes of Health (Fogarty International Center, NIH, USA). We thank the Clinical Research Unit from University Hospital – UFF for the partnership in the biochemical analysis.
REFERENCES
- Mafra D, Cuppari L, Cozzolino SM. Iron and zinc status of patients with chronic renal failure who are not on dialysis. J Ren Nutr. 2002;12:38–41.
- Bozalioglu S, Ozkan Y, Turan M, Simşek B. Prevalence of zinc deficiency and immune response in short-term hemodialysis. J Trace Elem Med Biol. 2005;18:243–249.
- Esfahani ST, Hamidian MR, Madani A, et al. Serum zinc and copper levels in children with chronic renal failure. Biol Trace Elem Res. 2006;124:103–109.
- Hsieh YY, Shen WS, Lee LY, Wu TL, Ning HC, Sun CF. Long-term changes in trace elements in patients undergoing insulin activity in genetically obese (ob/ob) mice. Biol Trace Elem Res. 2006;61:303–311.
- Kiziltas H, Ekin S, Erkoc R. Trace element status of chronic renal patients undergoing hemodialysis. Biol Trace Elem Res. 2008;124(2):103–109.
- Lobo JC, Torres JPM, Fouque D, Mafra D. Zinc deficiency in chronic kidney disease: is there a relationship with adipose tissue and atherosclerosis? Biol Trace Elem Res. 2010;135:16–21.
- Molavi B, Mehta JL. Oxidative stress in cardiovascular disease: molecular basis of its deleterious effects, its detection, and therapeutic considerations. Curr Opin Cardiol. 2010;19:488–493.
- Pedrosa AM, Faine LA, Grosso DM, de Las Heras B, Boscá L, Abdalla DS. Electronegative LDL induction of apoptosis in macrophages: involvement of Nrf2. Biochim Biophys Acta. 2010;1801:430–437.
- Sanchez-Quesada JL, Benıtez S, Ordonez-Llanos J. Electronegative low-density lipoprotein. Curr Opin Lipidol. 2004;15(3):329–335.
- Chen HH, Hosken BD, Huang M, et al. Electronegative LDL from familial hypercholesterolemic patients are physico-chemically heterogeneous but uniformly proapoptotic. J Lipid Res. 2007;48:177–184.
- Oliveira JA, Sevanian A, Rodrigues RJ, Apolinário E, Abdalla DS. Minimally modified electronegative LDL and its autoantibodies in acute and chronic coronary syndromes. Clin Biochem. 2006;39:708–714.
- Yang CY, Chen HH, Huang MT, et al. Pro-apoptotic low-density lipoprotein subfractions in type II diabetes. Atherosclerosis. 2007;193:283–291.
- Lobo J, Santos F, Grosso D, et al. Electronegative LDL and lipid abnormalities in patients undergoing hemodialysis and peritoneal. Nephron Clin Pract. 2008;108(4):298–304.
- Keys A, Fidanza KMJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–343.
- Rodriguez MP, Narizano A, Demczylo V, Cid A. A simple method for the determination of zinc human plasma levels by flame atomic absorption spectrophotometry. At Spectrosc. 1989;10(2):68–70.
- Gibson RS. Principles of Nutritional Assessment. New York: Oxford University; 1990:543–553.
- Faulin TES, Sena KCM, Telles AER, Grosso DM, Faulin EJB, Abdalla DS. Validation of a novel ELISA for measurement of electronegative low-density lipoprotein. Clin Chem Lab Med. 2008;46(12):1769–1775.
- Freedman JE, Loscalzo J. Platelet–monocyte aggregates: bridging thrombosis and inflammation. Circulation. 2002;105:2130–2132.
- Anderson TJ. Nitric oxide, atherosclerosis and the clinical relevance of endothelial dysfunction. Heart Fail Rev. 2003;8:71–86.
- Zou MH, Shi C, Cohen RA. Oxidation of the zinc thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826.
- Beattie JH, Kwun IS. Is zinc deficiency a risk factor for atherosclerosis? Br J Nutr. 2004;91:177–181.
- Torra IP, Chinetti G, Duval C, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors: from transcriptional control to clinical practice. Curr Opin Lipidol. 2001;12:245–254.
- Reiterer G, MacDonal R, Browning JD, et al. Zinc deficiency increases plasma lipids and atherosclerotic markers in LDL-receptor-deficient mice. J Nutr. 2005;135:2114–2118.
- Mocchegiani E, Costarelli L, Giacconi R, et al. Nutrient-gene interaction in ageing and successful ageing. A single nutrient (zinc) and some target genes related to inflammatory/immune response. Mech Ageing Dev. 2006;127:517–525.
- Prasad A. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008;43:370–377.
- Prasad AS, Beck FW, Bao B, et al. Zinc supplementation decreases incidence of infections in the elderly effect of zinc in generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837–844.
- Shen H, Oesterling E, Stromberg TM, MacDonald R, Hennig B. Zinc deficiency induces vascular pro-inflammatory parameters associated with NF-kappa B and PPAR signaling. J Am Coll Nutr. 2008;27:577–587.
- Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB. Nutrition. 2011;27:816–823.
- Wilkins GM, Leake DS. The oxidation of low density lipoprotein by cells or iron is inhibited by zinc. FEBS Lett. 1994;341:259–262.
- Meerarani P, Ramadass P, Toborek M, Bauer HC, Bauer H, Hennig B. Zinc protects against apoptosis of endothelial cells induced by linoleic acid and tumor necrosis factor alpha. Am J Clin Nutr. 2000;71:81–87.
- Bao B, Prasad AS, Frances WJB, et al. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects a potential implication of zinc as an atheroprotective agent. Am J Clin Nutr. 2010(91):1634–1641.
- Benítez S, Camacho M, Bancells C, Vila L, Sánchez-Quesada JL, Ordóñez-Llanos J. Wide proinflammatory effect of electronegative low-density lipoprotein on human endothelial cells assayed by a protein array. Biochim Biophys Acta. 2006;1761(9):1014–1021.
- Hennig B, Meerarani P, Toborek M, McClain CJ. Antioxidant-like properties of zinc in activated endothelial cells. J Am Col Nut. 1999;18(2):152–158.
- Davis SR, Cousins RJ. Metallothionein expression in animals: a physiological perspective on function. J Nutr. 2000;130:1085–1088.
- Mocchegiani E, Giacconi R, Cipriano C, et al. Zinc, metallothioneins, and longevity effect of zinc supplementation: zincage study. Ann NY Acad Sci. 2007;1119:129–146.
- Mariani E, Mangialasche F, Feliziani FT, et al. Effects of zinc supplementation on antioxidant enzyme activities in healthy old subjects. Exp Gerontol. 2008;43:445–451.
- Balsam A, El Kossi MM, Lord R, El Nahas AM. Cardiovascular disease on hemodialysis: predictors of atherosclerosis and survival. Hemodialysis Int. 2009;13:278–285.
- Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Inflammatory markers, lipoprotein components and risk of major cardiovascular events in 65,005 men and women in the Apolipoprotein Mortality Risk study (AMORIS). Atherosclerosis. 2010;213:299–305.
- Fan ZX, Hua Q, Li YP, Liu RK, Yang Z. Interleukin-6, but not soluble adhesion molecules, predicts a subsequent mortality from cardiovascular disease in patients with acute ST-segment elevation myocardial infarction. Cell Biochem Biophys. 2011;61:443–448.
- Pararameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20(2):87–103.
- Zoccali C, Mallamaci F, Tripepi G, Cutrupi S, Pizzini P. Low triiodothyronine and survival in end-stage renal disease. Kidney Int. 2006;70(3):523–528.
- Fellah H, Hammami MB, Fek M, et al. Predictors for cardiovascular morbidity and overall mortality in Tunisian ESRD patients: a six year prospective study. Clin Biochem. 2009;42(7–8):648–653.
- Kuragano T, Itoh K, Shimonaka Y, et al. Hepcidin as well as TNF-α are significant predictors of arterial stiffness in patients on maintenance hemodialysis. Nephrol Dial Transplant. 2011;26(8):2663–2667 10.
- Roberts MA, Polkinghorne KR, McDonal SP, Ierin FL. Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am J Kidney Dis. 2011;58:654–72.
- Iseki K. Role of chronic kidney disease in cardiovascular disease: are we different from others? Clin Exp Nephrol. 2011;15(4):450–455.
- Afsar B, Elsurer R, Akgul A, Sezer S, Ozdemir FN. Factors related to silent myocardial damage in hemodialysis patients. Ren Fail. 2009;31(10):933–941.