Abstract
Accumulating evidence suggests an association between body volume overload and inflammation in chronic kidney diseases. The purpose of this study was to evaluate the effect of dialysate sodium concentration reduction on extracellular water volume, blood pressure (BP), and inflammatory state in hemodialysis (HD) patients. In this prospective controlled study, adult patients on HD for at least 90 days and those with C-reactive protein (CRP) levels ≥ 0.7 mg/dL were randomly allocated into two groups: group A, which included 29 patients treated with reduction of dialysate sodium concentration from 138 to 135 mEq/L; and group B, which included 23 HD patients not receiving dialysate sodium reduction (controls). Of these, 20 patients in group A and 18 in group B completed the protocol study. Inflammatory, biochemical, hematological, and nutritional markers were assessed at baseline and after 8 and 16 weeks. Baseline characteristics were not significantly different between the two groups. Group A showed a significant reduction in serum concentrations of tumor necrosis factor-α, and interleukin-6 over the study period, while the BP and extracellular water (ECW) did not change. In Group B, there were no changes in serum concentrations of inflammatory markers, BP, and ECW. Dialysate sodium reduction is associated with attenuation of the inflammatory state, without changes in the BP and ECW, suggesting inhibition of a salt-induced inflammatory response.
INTRODUCTION
Cardiovascular diseases (CVD) are the major cause of death in hemodialysis (HD) patients, with a mortality rate that is 10- to 20-fold higher than that of the general population.Citation1,Citation2 The mechanisms proposed for the genesis of CVD in HD patients include hypervolemia, hyperhomocysteinanemia, and secondary hyperparathyroidism in addition to traditional factors such as diabetes, hypertension, sedentary lifestyle, lipid disorders, and others.Citation3 Furthermore, inflammation has been identified as a risk factor for atherosclerosis in these patients.Citation4 Some potential causes of inflammation in HD patients are blood exposure to dialysis membranes, non-sterile dialysate use, and retention of cytokines, acidosis, and non-apparent infections.Citation5,Citation6
Inflammation and extracellular volume (ECV) expansion are common in HD patients. There is accumulating evidence that fluid overload may be associated with the inflammatory responses.Citation7,Citation8 Ortega et al. observed that in chronic kidney patients, volume expansion assessed by atrial natriuretic peptide was predictive of inflammation.Citation9 In peritoneal dialysis (PD) patients, ECV was independently associated with inflammation.Citation10 Among PD patients those with circulatory congestion, have malnutrition and higher median CRP levels.Citation8 Niebauer et al.Citation11 found that in patients with congestive heart failure, those with peripheral edema had significantly higher concentrations of endotoxins, and after diuretic treatment, endotoxin concentrations were significantly reduced. These authors suggested that intestinal wall edema associated with volume expansion would favor the translocation of bacterial endotoxins and trigger an inflammatory response.
Dialysate sodium concentrationCitation12,Citation13 is correlated with interdialytic weight gain (IDWG) and blood pressure (BP) control. We hypothesized that diffusive sodium loss affects the inflammatory state. To the best of our knowledge, no intervention study has assessed the effect of low-dialysate sodium on the inflammatory state in dialysis patients; therefore, the purpose of this study was to evaluate the effect of dialysate sodium concentration reduction on extracellular water (ECW), BP, and inflammatory markers in HD patients.
METHODS
The Institutional Research Ethics Committee approved the study protocol, and the patients signed an informed consent. The study included patients aged ≥18 years on HD for at least 90 days. Inflammation was defined as CRP levels ≥ 0.7 mg/dL. This cut off was obtained from the CRP median levels of all patients (n = 119) treated with HD in our Dialysis Unit, at baseline. Exclusion criteria were acute inflammatory processes, chronic inflammatory diseases, antibiotic use within the past 2 months, malignancies, and central venous catheter use.
Patients were randomly assigned by drawing lots, and allocated into two groups as follows: group A (n = 29), which was treated with a reduced dialysate sodium concentration from 138 to 135 mEq/L, and group B (controls, n = 23), in which the dialysate sodium concentration remained at 138 mEq/L. No other changes in dialytic prescription, as well as in dietary and medical recommendations were made. All patients were monitored by the same clinical staff throughout the study and were followed-up for 16 consecutive weeks. At baseline, and on the week 8th, and 16th weeks, demographical, clinical, laboratory, and nutritional data were assessed.
Clinical and demographical data (underlying renal disease, time on dialysis, sex, age, presence of diabetes, smoking status, medications used, BP, IDWG, and the occurrence of intradialytic hypotension and muscle cramps) were retrieved from the patients’ medical records. Hypotension was defined as the presence of BP levels lower than 90 × 60 mmHg. Systolic and diastolic BP was estimated by the average of the last 10 routine pre-dialysis measurements. IDWG was determined based on the changes in body weight between the end of HD and return to next session, considering the mean of the last 10 sessions.
Pre-dialysis blood samples were collected for the measurement of biochemical [serum albumin, sodium, creatinine, urea, glucose, cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and bicarbonate], inflammatory serum CRP, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6), and hematological markers (hematocrit, hemoglobin, and total lymphocyte count). TNF-α and IL-6 levels were determined by ELISA using commercial kits (R&D® Systems, Minneapolis, MN). The remaining tests were performed using standard methods.
Single-frequency bioelectrical impedance analysis (BIA) measurements were performed 30 min after HD sessions. The BIA device (Biodynamics® analyzer model 450) measures resistance (ohms) and reactance (ohms) directly and stores the information. The information was used by an internal microprocessor to perform subsequent calculations of total body water, intracellular water, and ECW volumes according to previously validated equations.Citation14,Citation15 Dietary sodium intake (g/day) was based on 72-h alimentary registry.
All patients were dialyzed three times per week, for 3.5–4 h per session, using low-flux polysulfone dialyzers and dialysate with bicarbonate buffer. Prescribed dialysis doses (Kt/V) were at least 1.4.
Results are expressed as the mean ± standard deviation, median (interquartile range), or percentage, as appropriate. Basal characteristics were analyzed by the unpaired t test, Mann–Whitney U test, or chi-square test. Parametric data were analyzed by ANOVA for repeated measures, whereas nonparametric data were assessed by the Friedman test. The significance was set at p < 0.05. All statistical tests were performed using the SPSS 16.0 software (SPSS, Inc.).
RESULTS
Fifty-two subjects enrolled, between April 2007 and February 2009, were randomly allocated into two groups as follows: group A, included 29 patients treated with a reduced dialysate sodium concentration from 138 to 135 mEq/L; and group B, included 23 subjects, controls. During the follow-up, nine subjects in group A and five subjects in group B were excluded because of acute infections. Therefore, 38 patients, 20 in group A and 18 in group B completed the protocol study. No significant difference was observed between the groups regarding basal characteristics ().
Table 1. Patient´s baseline characteristics.
Dietary sodium intake did not vary significantly in both the groups during the follow-up (baseline, 8th, and 16th week 16). Mean sodium intake (g/day) were 9.02 ± 0.9, 9.02 ± 1.6, and 8.71 ± 0.8 in group A (p = 0.81); and 9.54 ± 1.6, 9.33 ± 1.2, and 9.24 ± 1.28 in group B (p = 0.64).
Systolic and diastolic BP and IDWG showed no significant changes during the follow-up in both the groups. The means of systolic BP (mmHg) were 142.80 ± 20.45, 139.70 ± 23.2, and 137.20 ± 19.58 in group A (p = 0.39) and 142.33 ± 19.30, 148.50 ± 19.56, and 149.22 ± 20.44 in group B (p = 0.17). The diastolic BP were 76.34 ± 17.7, 73.5 ± 18.5, and 79.39 ± 10.22 in group A (p = 0.39) and 84.30 ± 13.10, 85.40 ± 11.00, and 83.60 ± 22.90 in group B (p = 0.73). The medians of IDWG (kg) were 2.26 (2.02; 2.79), 2.02 (0.75; 3.03), and 2.42 (2.02; 2.83) in group A (p = 0.15) and 2.64 (1.78; 3.50), 2.34 (1.84; 2.92), and 2.79 (1.44; 3.22) in group B (p = 0.11).
All patients received recombinant human erythropoietin, and the proportion of patients treated with iron hydroxide remained unchanged in both the groups throughout the study. During the follow-up, the proportion of subjects using statins or angiotensin-converting enzyme inhibitors (ACEI) (p = 0.19) and the median number of hypertensive classes did not statistically differ between the groups (p = 0.17).
The delivery dialysis dose (Kt/V) results at baseline, 8th, and 16th weeks were 1.36 ± 0.29, 1.35 ± 0.3, and 1.40 ± 0.29 in group A (p = 0.74) and 1.37 ± 0.15, 1.37 ± 0.24, and 1.45 ± 0.23 in group B (p = 0.20).
During the follow-up, 15 patients of group A and six patients of group B had experienced at least one episode of intradialytic hypotension. Therefore, the frequency of hypotension tended to be higher in group A (p = 0.07). Muscle cramps were reported by 15 patients of group A and by four patients of group B (p = 0.025).
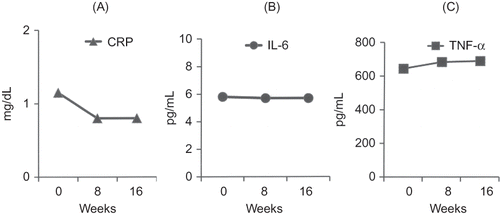
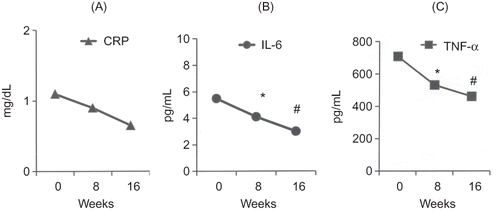
In group A, significant reductions of TNF-α and IL-6 levels were observed between the baseline and 8th week and between 8th and 16th week. In group B, no significant changes in these inflammatory markers levels were observed. Biochemical and hematological markers and CRP levels did not change significantly in either group, between any two assessment points (Figures 1 and 2, and ). BIA measurements did not change significantly in both the groups ().
Table 2. Hematological, serum biochemical, and inflammatory markers in group A (n = 20).
Table 3. Hematological, serum biochemical, and inflammatory markers in group B (n = 18).
Table 4. Bioelectric impedance (BIA) measurements in groups A (n = 20) and B (n = 18).
DISCUSSION
The results of this study showed that dialysate sodium reduction was associated with a reduction of inflammatory markers concentrations, while body volume markers, IDWG, and BP remained unchanged. It is possible that the intervention using dialysate sodium concentration reduction without dietary sodium restriction was not sufficient to achieve a reduction of these parameters. Interestingly, in a recent study, Shah and Davenport showed that the reduction of the dialysate from 140 to 136 mEq/L has minimal effects on BP control in young male patients, suggesting that this strategy is not sufficient to decrease BP in HD patients.Citation16 The authors suggest that additional dietary sodium restriction would be necessary to improve the pressure control. These data are compatible with the results of the present study.
Another possibility to explain our findings would be an additional source of sodium to patients in the treated group during episodes of intradialytic hypotension requiring therapy, which were more frequent in this group than in the control group. This additional amount of sodium could blunt the effects on IDWG, ECW, and BP.
Classically, it is thought that sodium retention is physiologically associated with increasing ECW, through a mechanism in which sodium and other ions, such as chloride, act osmotically to attract water into the extracellular space. This view has been questioned, suggesting that large amount sodium can be accumulated without water retention. Heer et al.Citation17 reported, in healthy adult humans, that sodium overload was not followed by the expected increases in ECW and body weight. Similarly, in young American females, changes in dietary sodium content were not associated with variations in serum sodium and body weight.Citation18 Bone, cartilage, skin, and connective tissue are important sodium reservoirs. Titze,Citation19 in a recent review, proposed that in these tissues, sodium ions would bind to extracellular matrix components, such as glycosaminoglycans, which are negatively charged, forming a “third space” with osmotically inactive sodium. Schaffhubber et al.Citation20 showed that rats with dietary sodium overload accumulated approximately 40% of the sodium in the skin, with mild or no increases in water content in this tissue; in the same experiment, there was an increase of glycosaminoglycan content in the skin. Indeed, in rats receiving low-sodium diets there was mobilization of osmotically inactive sodium associated with a decrease in glycosaminoglycan content in the skin.Citation20 Thus, it is possible that in our study the reduction of dialysate sodium concentration induced osmotically inactive sodium mobilization or, in other words, loss of sodium without water.
However, our findings make it somewhat difficult to interpret the mechanisms involved in the attenuation of inflammatory state. In this study, IL-6 and TNF-α serum concentrations decreased in patients treated with low-sodium dialysate, independently of ECW reduction. This suggests an additional mechanism by which sodium can promote an inflammatory response. Some evidence supports the hypothesis that sodium induces the gene expression of inflammatory response mediators. Human peripheral blood mononuclear cells exposed to hyperosmolar conditions by the addition of sodium chloride show increased gene expression of IL-1a, IL-1b, and IL-8, and increased phosphorylation of mitogen-activated protein kinase (p38 MAPK). Therefore, the link between inflammation and salt might include hyperosmolar sodium chloride, triggering p38 MAPK phosphorylation, and stimulating the synthesis of inflammatory cytokines.Citation21–Citation23
In this study, comparing baseline characteristics between the two groups there was a greater proportion of patients using statin and ACEI use in the control group, although these differences did not reach statistical significance. Considering that both statins and ACEI independently have anti-inflammatory effects,Citation24,Citation25 the effect of sodium reduction on inflammation was all the more impressive. Furthermore, the difference in statin use may be related to serum triglycerides profile over the study period, which trended to increase in the intervention group and to decrease in the control group.
This study has some limitations. In particular, we had a great exclusion rate (26.9% over 16 weeks) due to acute infections, and in consequence a small number of subjects completing the study protocol. This rate is seemingly greater than that observed by Aslam et al.Citation26 who reported a rate of infection-free patients of 45% among incident HD subjects followed-up by a median time of 18 months. Indeed, direct measurements of dialysate sodium concentrations were not performed, and there was not an accurate method to evaluate body water beyond BIA measurements. The main strength of our study is its prospective and randomized design. To our knowledge, this is the first to examine the effects of dietary sodium restriction alone on BP, body volume, and inflammation in HD patients.
In conclusion, the results of this prospective randomized interventional study show that reduction of dialysate sodium concentration is associated with inflammatory state attenuation in HD patients, and suggests that sodium plays an independent role in the genesis of inflammation in these patients. Therefore, reduction of dialysate sodium concentration seems to provide an effective strategy to improve the HD patient’s prognosis, particularly, in terms of cardiovascular events. However, the elevated frequency of hypotensive episodes during dialysis procedure deserves particular attention; it may be an important limitation for its widespread clinical use.
Figure 1. Evolution of serum inflammatory markers in adult hemodialysis (Group A, treated group, n = 20) patients treated by sodium dialysate reduction from 138 to 135 mEq/L. (A) CRP = C-reactive protein, (B) IL-6 = interleukin 6, and (C) TNF-α = alpha tumor necrosis factor. Note: *p < 0.05 versus baseline (week 0), #p < 0.05 versus week 8.
ACKNOWLEDGEMENTS
The authors thank the Research Group of Botucatu Medical School-UNESP for the methodological analysis and Janete Soares for her technical support.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The authors thank the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for its financial support.
REFERENCES
- Zoccali C, Mallamaci F, Tirpepi G. Inflammatory proteins as predictors of cardiovascular disease in patients with end–stage renal disease. Nephrol Dial Transplant. 2004;19(Suppl. 5):67–72.
- Stenvinkel P. Inflammation in end-stage renal failure: could it be treated? Nephrol Dial Transplant. 2002;17(Suppl. 8):33–38.
- Santoro A, Mansini E. Cardiac effects of chronic inflammation in dialysis patients. Nephrol Dial Transplant. 2002;17(Suppl. 8):10–15.
- Stenvinkel P, Heimburger O, Paultre F Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911.
- Yuen D, Chab CT. Inflammation, cardiovascular disease and nocturnal hemodialysis. Curr Opin Nephrol Hypertens. 2005;14:538–542.
- Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr. 2004;80:299–307.
- Pecoits-Filho R, Lindholm B, Stenvinkel P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome: the heart of the matter. Nephrol Dial Transplant. 2002;17(Suppl.11):S28–S31.
- Wang AY, Sea MM, Tang N Energy intake and expenditure profile in chronic peritoneal dialysis patients complicated with circulatory congestion. Am J Clin Nutr. 2009;90:1179–1184.
- Ortega O, Gallar P, Munoz M Association between C-reactive protein levels and N-terminal natriuretic peptide in predialysis patients. Nephrol Clin Pract. 2004;97(4):123–124.
- Ávila Díaz M, Venturra MJ, Valle D Inflammation and extracellular volume expansion and related to sodium and water removal in patients on peritoneal dialysis. Perit Dial Int. 2006;26:574–580.
- Niebauer J, Volk HD, Kemp M Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353:1838–1842.
- Ozkahya M, Ok E, Cirit M Long-term survival rates in hemodialysis patients treated with strict volume control. Nephrol Dial Transplant. 2006;21:3506–3513.
- Paula FM, Peixoto AJ, Pinto LV, Dorigo D, Patricio PJM, Santos SFF. Clinical consequences of an individualized dialysate sodium prescription in hemodialysis patients. Kidney Int. 2004;66:1232–1238.
- Kushner RF, Schoeller DA. Estimation of total body water by bioelectrical impedance analysis. Am J Clin Nutr. 1986;44:417–424.
- Cohn SH, Vaswani AN, Yasumura S, Yuen K, Ellis KJ. Assessment of cellular mass and lean body mass by noninvasive nuclear techniques. J Lab Clin Med. 1985;105:305–311.
- Shah A, Davenport A. Does a reduction in dialysate sodium improve blood pressure control in hemodialysis patients? Nephrology. 2012;17:358–363.
- Heer M, Frings-Meuthen P, Titze J Increasing sodium intake from a previous low or high intake affects water, electrolyte and acid-base balance differently. Br J Nutr. 2009;101:1286–1294.
- Titze J, Maillet A, Lang R Long-term sodium balance in humans in a terrestrial space station simulation study. Am J Kidney Dis. 2002;40:508–516.
- Titze J. Water-free sodium accumulation. Semin Dial. 2009;22(3):253–255.
- Schafflhuber M, Volpi N, Dahlmann A 1. Mobilization of osmotically inactive Na+ by growth and by dietary salt restriction in rats. Am J Physiol Renal Physiol. 2007;292:F1490–F1500.
- Dinarello CA. Hyperosmolar sodium chloride, p38 mitogen activated protein and cytokine-mediated inflammation. Semin Dial. 2009;22:256–259.
- Shapiro L, Dinarello CA. Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci USA. 1995;92:12230.
- Shapiro L, Dinarello CA. Hyperosmotic stress as a stimulant for proinflammatory cytokine production. Exp Cell Res. 1997;231:354–362.
- Deng J, Wu Q, Liao Y, Huo D, Yang Z. Effect of statins on chronic inflammation and nutrition status in renal dialysis patients: a systematic review and meta-analysis. Nephrology (Carlton). 2012;17:545–551.
- Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovasc Drug Rev. 2006;24:33–50.
- Aslam N, Bernardini J, Fried L, Burr R, Piraino B. Comparison of infectious complications between incident hemodialysis and peritoneal dialysis patients. Clin J Am Soc Nephrol. 2006;1:1226–1233.