Abstract
Hepatorenal syndrome (HRS) represents a complication of the end-stage liver cirrhosis. The aim of the present study was to analyze concentrations of nitrates and nitrites (NO2 + NO3) and L-arginine in patients with liver cirrhosis and HRS as a possible predictive marker for the development of HRS. The research was performed in a group of 28 patients with cirrhosis and HRS, a group of 22 patients suffering from cirrhosis without HRS and a control group comprised of 42 healthy voluntary blood donors. In patients with end-stage alcoholic liver cirrhosis, with HRS, the concentrations of NO2 + NO3 increased and correlated with the degree of cirrhosis progression, compared to patients without HRS and significantly higher compared to the control group. The level of NO2 + NO3 was in a positive correlation with the degree of liver damage de Ritis coefficient (HRS = 0.72; cirrhosis: = 0.55; control = −0.10). Significant positive correlation was found between NO2 + NO3 concentration and inflammatory marker C-reactive protein (HRSC = 0.75; cirrhosis = 0.70, control = −0.25). The correlation between NO2 + NO3 concentration and creatinine concentration in patients with HRS was significantly higher compared to patients without HRS (HRS = 0.82; cirrhosis = 0.32; control = −0.25). By using binary regression analysis, on the basis of clinical criteria of HRS diagnosis, the strongest independent positive predictor for HRS development was NO2 + NO3, associated with 45.02 times higher incidence of HRS, compared to arginine (12.7 times higher incidence), creatinine (13.1 times higher incidence), and AST/ALT ratio (10.55 higher incidence of HRS). Since the determination of NO2 + NO3 represents a reliable and easily applicable method, it may be used as an early predictive marker for HRS development.
INTRODUCTION
Hepatorenal syndrome (HRS) is a potentially reversible syndrome which develops into chronic liver insufficiency. It is characterized by kidney dysfunction and severe changes in systemic circulation. Reduction in kidney function is a consequence of the reduction in blood flow through the kidneys, manifested as the reduction in glomerular filtration. Among the pathogenic mechanisms are the activation of specific vasoconstrictor system with the activation of sympathetic system, renin–angiotensin system, and vasopressin.Citation1 Based on a new consensus concerning definition, diagnosis, and treatment of HRS, the International Ascites Club set up a new criterion for HRS diagnosis.Citation2
Nitric oxide (NO) is an intercellular signaling molecule, synthesized in the endothelial cells of blood vessels, macrophages, and other mammalian cells. It is synthesized from L-arginine by the enzyme nitric oxide synthase (NOS). The three isoforms of the NOS enzyme are documented: neuronal (nNOS), inducible or inflammatory (iNOS), and endothelial (eNOS).Citation3 NO participates in the regulation of vascular tone, in the prevention of leucocyte adhesion, platelets aggregation, and proliferation of smooth muscle cells. Beside this, NO is involved in neurotransmission. It exerts the antioxidative and the antiapoptotic properties (via maintenance of membrane potential in mitochondria, reverse inhibition of the release of cytochrome C and inactivation of caspase 3). It is also able to reduce the TNF-α toxicity. But in the presence of excess of reactive oxygen species , toxic peroxinitrite may be formed. Peroxinitrite may induce DNA damage by activation of nuclear PARP enzyme, by participation in lipid peroxidation and by inhibition of enzymes via nitro-oxidation and nitrosylation.Citation4–Citation6 Nitric oxide in liver sinuses maintains the microvascular perfusion in the liver. In liver failure, the ability of liver to meet the metabolic needs of the body is reduced. An important pathogenic mechanism by which the liver compensates the disorders of circulatory system is found in an increased production of NO under the effect of iNOS induced by inflammatory cytokines and CRP.Citation7 Endotoxins can activate macrophages in the liver and blood. In situations of increased NO and ROS production, NO anion (NO-) and superoxide anion (O2-) form peroxynitrite anion and hydroxyl radicals. Through the process of oxidation and nitration, peroxynitrites can modify proteins, lipids, carbohydrates, and nucleic acids of cells. Modification of these biomolecules causes changes in their structure and function.Citation8 In this way, liver tissue and vascular endothelium are damaged. Irreversible damage to hepatocytes and endothelial dysfunction lead to disorders of chemodynamics and development of portal hypertension.Citation9 Farzaneh-Far,Citation10 documented that the disorder in the regulation of the control of NO system synthesis plays a key role in the pathogenesis of chronic liver disease.Citation10 In patients with cirrhosis, the production of NO2 + NO3 is involved in the pathogenesis of abnormalities of chemodynamics of the liver and kidneys.Citation11 It is assumed that NO2 + NO3, synthesized by the action of iNOS and/or eNOS, represents key pathogenic factor in the development of clinical complications of portal hypertension, such as: hepatopulmonary syndrome, portal cardiomyopathy, HRS, and hepatic encephalopathy.Citation12 Our previous research has shown that in patients with alcoholic cirrhosis, arginine metabolites, such as ADMA and SDMA, can correlate with the degree of kidney failure and the development of HRS.Citation13
The level of NO2 + NO3 in the plasma of patients with alcoholic cirrhosis with and without HRS was not explored. Our hypothesis is that the level of NO2 + NO3 and L-arginine level may be the possible markers in early diagnostics of HRS syndrome. Therefore, the aim of this research was to estimate the level of NO2 + NO3 in the plasma of patients with cirrhosis with and without HRS, as well as to examine the significance of NO2 + NO3 with respect to the severity of liver disease and degree of HRS development in patients with cirrhosis.
PATIENTS AND METHODS
The research included two study groups: a group of patients with cirrhosis, divided based on the presence of HRS and a group of healthy examinees. The patients with cirrhosis were hospitalized in the Clinic for gastroenterology Clinical Center Nis (Serbia). The group with cirrhosis included 50 patients who had been consuming alcohol for over 10 years. All of them were males, average age 51.13 ± 23 years. All patients had end-stage alcoholic cirrhosis, with moderate to severe ascites. HRS was diagnosed in 28 patients with cirrhosis, while 22 patients did not have HRS. Catheters were inserted in patients with HRS. Control group included 42 healthy examinees that were voluntary blood donors from transfusiology ward at the Clinical Center Nis. All participants from the control group were male, average age 49.76 ± 9.7 years, with normal laboratory findings. Cirrhosis was also diagnosed by liver biopsy. Presence of ascites was confirmed by diagnostic paracentesis. Hepatorenal syndrome was diagnosed in accordance with the latest criteria suggested by the International Ascites Club.Citation14 The criteria included: cirrhosis with ascites, low glomerular filtration, serum creatinine over 133 μmol/L (over 1.5 mg/dL), proteinuria less than 500 mg/day, absence of shock, absence of bacterial infection, loss of fluid, impaired kidney function after cessation of diuretic treatment (serum creatinine value which remains at the level of ≥ 133 μmol/L for at least 48 hours, after administration of albumin dose 1 to 100 g/kg a day), treatment without nephrotoxic drugs, absence of parenchymal renal disease (patient does not have proteinuria > 500 mg/day, no microhematuria >50 erythrocytes, no pathological findings of ultrasound examination of the kidneys).
The research was conducted in accordance with the ethical standards of the Human Experimentation Committee. The study is prospective, conducted in accordance with the Ethics Committee and preceded by informed consent from every patient.
General biochemical parameters were obtained from serums taken from participants using standard biochemical IFCC methods (OLYMPUS-AU680). NO2 + NO3 concentrations were determined using Navarro–Gonzalez semiautomated method, based on diazotization of sulfanilic acid.Citation15 l-arginine concentration was determined by using HPLC system.
STATISTICS
The results obtained by the research were presented as average values with standard deviations (95% CI for mean). The significance of the differences was determined by ANOVA test. Post hoc Dunnett’s T3 analysis and Tuckey test were also performed. Statistically significant difference was accepted with risk of p < 0.05. The relation between the tested variables was determined by linear regression analysis and goodness of fit analysis, as well as by Pearson’s correlation coefficient. The association between the monitored biochemical markers and the development of HRS in patients with alcoholic liver cirrhosis was tested using binary logistic regression analysis (Enter model).
RESULTS
Basic demographic and clinical laboratory analyses were shown in . The group of patients with HRS had significantly more severe liver damage. This was concluded from the value of de Ritis coefficient (AST/ALT) which was considerably higher in patients with HRS, compared to patients without HRS and compared to healthy examinees. Synthetic liver function was significantly reduced in patients with HRS, as well as in patients without HRS, compared to healthy examinees. This was reflected by the level of albumins in serum. Excretory liver function was greatly reduced in patients with HRS, compared to patients without HRS and healthy examinees. This could be observed from the level of total bilirubin and from a significant increase of indirect bilirubin. The standard parameters of renal failure, urea, and creatinine level were significantly higher in patients with HRS, compared to patients without HRS and healthy examinees. showed the correlation between NO2 + NO3 and AST/ALT in groups of patients with cirrhosis, with and without HRS. In cirrhosis, the level of NO2 + NO3 in serum increased along with the increase of AST/ALT ratio (C = 0.55; *p < 0.05). With the progression of cirrhosis and the development of HRS, with manifested renal failure, the association between NO2 + NO3 and AST/ALT increased (C = 0.72). However, negative association was shown between NO2 + NO3 and the level of CRP in the control group. In cirrhosis, the level of NO2 + NO3 increased with an increase in CRP level (C = 0.70). With the progression of cirrhosis and the development of HRS in renal failure, the association between NO2 + NO3 and CRP positively correlated (C = 0.75; *p <0.05) (). Significant negative correlation was found between NO2 + NO3 and creatinine concentration in the control group. In cirrhosis, the level of NO2 + NO3 in the serum increased, with an increase in creatinine concentration (C = 0.32; *p <0.05). With the progression of cirrhosis and development of HRS, when renal failure is manifested, the association between NO2 + NO3 and creatinine also increased (C = 0.82) (). shows the values obtained for NO2 + NO3, as well as for arginine in all the three groups. The lowest arginine values were obtained in groups with HRS. The level of NO2 + NO3 was significantly higher in groups with cirrhosis with and without HRS, compared to control. The association between the monitored biochemical markers and the development of HRS in patients with alcoholic liver cirrhosis was tested using logistic regression analysis (Enter model), whereby output variable of interest was marked 0–cirrhosis present and 1–developed HRS. The results are shown in . Binary regression analysis on the basis of clinical criteria of HRS diagnosis showed that the tested parameters may play a role in the development of HRS. The strongest independent positive predictor was shown by NO2 + NO3values, whose increase was associated with 45.02 times higher incidence of HRS, by arginine values, whose increase was associated with 12.7 times higher incidence of HRS, by creatinine values whose growth increases HRS by 13.1 times, and AST/ALT ratio whose increase was associated with 10.55 higher incidence of HRS ().
Figure 1. The association between NO2 + NO3 concentration and AST/ALT ratio in tested groups. (The relation between the tested variables was determined by linear regression analysis and goodness of fit analysis, as well as by Pearson’s correlation coefficient). NO2 + NO3 are expressed as μmol/L.
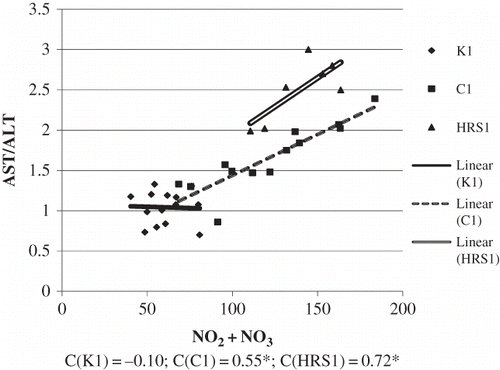
Figure 2. The association between NO2 + NO3 and CRP in tested groups. (The relation between the tested variables was determined by linear regression analysis and goodness of fit analysis, as well as by Pearson’s correlation coefficient). NO2 + NO3 are expressed as μmol/L and CRP as mg/L.
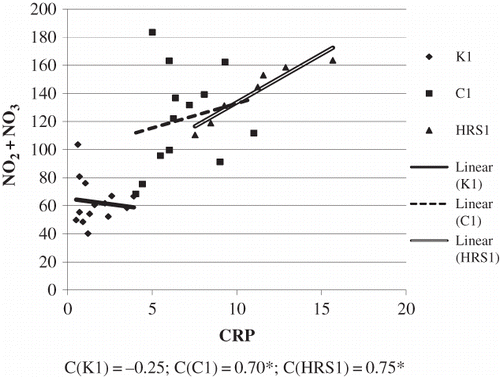
Figure 3. The association between NO2 + NO3 concentration and creatinine concentration in tested groups. (The relation between the tested variables was determined by linear regression analysis and goodness of fit analysis, as well as by Pearson’s correlation coefficient). NO2 + NO3 and creatinine are expressed as μmol/L.
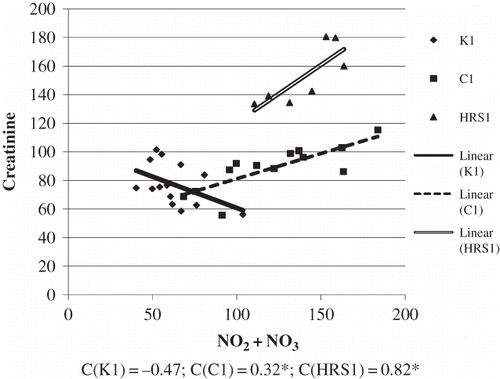
Table 1. Basic demographic clinical and biochemical characteristics of patients and healthy examinees.
Table 2. Arginine and NO2 + NO3 in the tested groups.
Table 3. Predictive value of analyzed markers in the development of HRS.
DISCUSSION
This study demonstrated that in chronic alcoholism, l-arginine metabolites can participate in liver and kidney damage. This was shown in our previous research, via arginine-methylated products ADMA and SDMA.Citation13 The liver has an important function in the metabolism of arginine and its metabolites. Obtained results demonstrated that groups of patients with cirrhosis with and without HRS had significantly increased level of NO2 + NO3, compared to control group. The results also show that in groups with cirrhosis, the level of NO2 + NO3 positively correlated with the degree of liver damage, i.e., with de Ritis coefficient. This could point to cause–effect relationship between nitric oxide (NO) metabolism, liver damage, and cirrhosis progression with the development of renal failure. Different values of NO2 + NO3 levels in groups with and without HRS correlated with the degree of fibrosis and inflammation in liver cirrhosis.Citation16 By disturbing the metabolism of various endogenous and exogenous compounds in the liver and producing ROS, alcohol consumption causes chronic oxidative liver damage. Oxidative stress contributes to pathogenesis of alcoholic liver damage, as well as to progression of alcohol-induced liver disease which results in alcoholic liver fibrosis.Citation17–Citation19 With the progression of alcoholic liver disease, overproduction of ROS may cause damage of proteins, enzymes, and lipids, as well as irreversible metabolic disturbances in the liver. In chronic alcoholic liver damage, ROS stimulate the activation of stellate cells and development of fibrogenesis.Citation20 Several different studies have shown that during chronic alcohol consumption, in class A alcoholic cirrhosis, as well as in decompensated B and C classes, the level of NO2 + NO3 was higher compared to the respective hepatitis B-induced cirrhosis. Contrary to our results, the study by Pârvul A. et al., did not find significantly increased level of NO2 + NO3 in patients with cirrhosis, compared to the level of NO2 + NO3 in control group.Citation21 The results of our research () were in accordance with the results obtained by Arkenau et al., where the level of NO2 + NO3 positively correlated with the plasma ALT activity.Citation22
The level of CRP was determined in order to estimate the degree of inflammation. In the group with HRS, the level of CRP was almost twice as high, compared to the CRP level in cirrhosis and almost eight times higher compared to the control group. Thus, it could be pointed out that inflammatory components of the disease were also associated with the activity of inducible NO synthase which significantly influences the inflammatory damage of liver functions and progression of renal failure. The research shows that during chronic alcohol consumption, the progression of cirrhosis and development of HRS was accompanied by significant positive correlation between NO2 + NO3 and CRP. The correlation between NO2 + NO3 and CRP may point out to the development of inflammation in the liver (). In severe oxidative stress and inflammation, the liver may redirect the normal metabolic pathway of l-arginine from the synthesis of polyamines towards the synthesis of pathological metabolites. By a process of ROS production and activation of iNOS, NO is quickly inactivated into peroxynitrites. L-Arginine concentration may be thus reduced. The expression of iNOS depends on the degree of inflammation of liver cirrhosis. In cirrhosis, iNOS activity is present in hepatocytes, Kupffer cells, endothelial cells of liver sinuses, as well as in the inflammatory cells of infiltrates of the portal tract and blood monocytes. In oxidative stress, by means of inflammatory cytokines and CRP, liver macrophages and blood monocytes induce iNOS in order to increase the production of NO and peroxynitrites.Citation23 The mechanism may be viewed in two ways, as self-regulatory, as well as a reactive systemic inflammation. Our results are in accordance with the results obtained by Kirkham et al., who have demonstrated that by the activation of macrophages, oxidative stress contributes to the development of inflammatory process, and that oxidative stress together with inflammation leads to liver failure.Citation24 Therefore, it could be suggested that liver was not responsible for the formation of NO, but that rather it could be caused by inflammation. In systemic inflammation, after the development of portal hypertension, increased iNOS activity can cause vascular collapse. The research by Kirkali G. et al., shows that the level of NO2 + NO3 is significantly correlated with the degree of liver inflammation, which is in line with the results obtained in our study.Citation24 The accumulation of peroxynitrite may disturb the normal function of endothelial cells and the synthesis of vasoactive substances, what may cause portal hypertension and its complications.Citation25,Citation26 Our research has shown a significantly higher level of NO2 + NO3 in HRS, compared to control group. In the patients with HRS, constant contact between blood and catheter endotoxins, due to urine retention may activate the immune-inflammatory cascade. Higher levels of circulating endotoxins in patients with cirrhosis have been documented to increase the expression of iNOS, leading to the excessive NO synthesis.Citation27
Regarding the kidney function, plasma creatinine level was almost two times higher in the HRS group, compared to the level in the group with cirrhosis, and significantly higher compared to the control group (). The results of the study also showed that in the group with HRS, the level of NO2 + NO3 significantly correlated with the level of creatinine (). The level of NO2 + NO3 was documented to correlate with the progression of kidney function damage.Citation28,Citation29 The results showed that the level of NO2 + NO3 was higher in decompensated cirrhosis with renal failure, compared to compensated cirrhosis.
The progression of cirrhosis intensifies oxidative stress. In the respiratory chain, increased leakage of electrons leads to mitochondrial dysfunction. Mitochondrial dysfunction causes reduction in ATP synthesis. Increased production of O2− with NO− anion creates toxic peroxynitrites. Peroxynitrites cause extended hypoperfusion of the kidneys and medullary hypoxia, which contributes to the damage of tubular cells of the kidneys.Citation30 Tubular epithelial cells are not capable of synthesizing glutathione, compared to other nephron segments, therefore tubular cells would be among the most susceptible ones to ROS production. Hypertrophy of tubular cells, beside glomerulosclerosis and interstitial fibrosis, causes progression of renal failure. But in cirrhosis, reduced glomerular filtration seems to be a major abnormality of renal failure. In patients with cirrhosis, kidney function is sensitive to changes in blood volume and to toxins. By activating the renin–aldosterone system and by vasopressins, glomerular filtration intensifies resorption of sodium in proximal tubules and reduces water clearance. In cirrhosis, prolonged hypoperfusion of the kidneys, renal medullary hypoxia, and high frequency of infective complications contribute to the damage of the renal tubules.Citation31,Citation32 Our results agree with the results obtained by Guarner et al., which confirm the significance of NO2 + NO3 in the damage of liver and kidneys, in patients with cirrhosis and disorder of kidney function.Citation33 In patients with chronic renal failure with catheters inserted, a rapid increase in uremic toxins was found. In uremic patients, increase in peroxynitrites may accelerate atherosclerosis. Atherosclerosis of kidney blood vessels may be a cause of morbidity and mortality in patients with chronic cirrhosis.Citation34,Citation35
The results obtained in our study may suggest that NO2 + NO3 can be formed either due to rapid l-arginine degradation or due to activation of inducible NO synthase during inflammation. However, oxidative stress and inflammation may induce its modification into toxic peroxynitrite which affects endothelial cells, particularly, glomerular capillaries. Our study showed that arginine concentration decreases with an increase in NO2 + NO3 concentration. In the group of patients with HRS, L-arginine concentration had the lowest values. This confirmed reduced liver function that could be in accordance with the results obtained by Cherla G. et al.Citation36
CONCLUSION
The level of NO2 + NO3 may be used as the strongest independent positive predictor for progression toward HRS, compared to L-arginine, creatinine, or AST/ALT ratio. Possible modification of eNOS and/or iNOS enzyme activity, which participate in NO synthesis, may represent a new treatment goal in order to reduce the progression of liver and kidney damage.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Gines P, Guevara M, Arroyo V, Rodes J. Hepatorenal syndrome. Lancet. 2003;362:1819–1827.
- Salerno F, Gerbes A, Gine’s P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318.
- Alderton W, Cooper C, Knowles R. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357: 593–615.
- Gow AJ, Luchsinger BP, Pawlovski JR, Singel DJ, Stampler JS. The oxihemoglobin reaction of nitric oxide. Proc Natl Acad Sci ASA. 1999;96:9027–9032.
- Kim M, Talanian V, Billiar R. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem. 1997;272:31138–31148.
- Feletou M, Vanhoutte M. Endothelial dysfunction: a multifaceted disorder. Am J Physiol Heart Circ Physiol. 2006;291:H985– 1002.
- Taylor BS, Alarcon L, Billiar TR. Inducible Nitric oxide synthase in the liver: regulation and function. Biochemistry (Mosc). 1998;63(7):766–781.
- Joshi S, Ferguson B, Han H, et al. Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. Proc Natl Acad Sci USA. 2002;6:10341–10346.
- Shah V, Haddad G, Garcia-Cardena G, et al Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J Clin Invest. 1997;100:2923–2930.
- Farzaneh-Far R, Moore K. Nitric oxide and the liver. Liver. 2001;21:161–174.
- Gines P, Renal SD. Failure in Cirrhosis. Review article N Engl J Med. 2009;361:1279–1290.
- Genesca J, Gonzalez A, Segura R, et al. Interleukin-6, nitric oxide, and the clinical and hemodynamic alterations of patients with liver cirrhosis. Am J Gastroenterol.1999;94:169–177.
- Nickovic V, Nikolic J, Djindjić N, et al. Diagnostical significance of dimethylarginine in the development of hepatorenal syndrome in patients with alcoholic liver cirrhosis. Vojnosan Pregled. 2012;69:686–691.
- Salerno F, Gerbes A, Gine’s P, Wong F, Arroyo V. Diagnosis prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318.
- Navarro-Gonzales JA, Garcia-Benayas C, Arenos J. Semiautomated measurement of nitrate in biological fluids. Clin Chem. 1998;44:679–682.
- Arkenau T, Stichtenoth O, Frolich C, Manns P, Boker H. Elevated nitric oxide levels in patients with chronic liver disease and cirrhosis correlate with disease stage and parameters of hyperdynamic circulation. Z Gastroenterol. 2002;40:907–913.
- Feletou M, Vanhoutte M. Endothelial dysfunction: a multifaceted disorder. Am J Physiol Heart Circ Physiol. 2006;291:H985–1002.
- Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306.
- Friedman L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250.
- Wu D, Cederbaum I. Alcohol oxidative stress, and free radical damage. Alcohol Res Health. 2003;27:277–284.
- Parvu1 A, Negrean V, Pleca-Manea1 L, Cosma A, Draghici A. Nitric oxide in patients with chronic liver diseases. Romanian J Gastroenterol. 2005;14:225–230.
- Capone F, Guerriero E, Sorice A, et al. Characterization of metalloproteinases, oxidative status and inflammation levels in the different stages of fibrosis in HCV patients. Clin Biochem. 2012;45:525–529.
- Mohammed N, El-Aleem S, Appleton I, et al Expression of nitric oxide synthase isoforms in human liver cirrhosis. Patology. 2003;200:647–655.
- Kirkham P. Oxidative stress and macrophage function: a failure to resolve the inflammatory response. Biochem Soc Trans. 2007;35:284–287.
- Kirkali G, Gezer S, Umur N, Ali M, Tankurt Z. Nitric oxide in chronic liver disease. Turk J Med Sci. 2000;30:511–515.
- Higashino H, Tabuchi M, Yamagata S, et al. Serum nitric oxide metabolite levels in groups of patients with various diseases in comparison of healthy control subjects. J Med Sci. 2010;10:1–11.
- Stenvinkel P, Heimburger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911.
- Turkay C, Yonem O, Arikan O, Baskin E. Nitric oxide and renal functions in liver cirrhosis. Turk J Gastroenterol. 2004;15:73–76.
- Porst M, Hartner A, Krause H, Hilgers KF, Veelken R. Inducible nitric oxide synthase and glomerular hemodynamics in rats with liver cirrhosis. Am J Physiol Renal Physiol. 2001;281:F293–F299.
- Granata S, Zaza G, Simone S, et al. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease.BMC Genomics. 2009;10:388.
- Martin Y, Gin SP, Schrier W. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Eng J Med. 1998;339:533–541.
- Albillos A, Rossi I, Cacha G, et al. Enhanced endothelium-dependent vasodilatation in patients with cirrhosis. Am J Physiol. 1995;268:G459–G464.
- Guarner C, Soriano G, Tomas A, et al. Increased serum nitrite and nitrate levels in patients with cirrhosis: relationship to endotoxemia. Hepatology. 1993;18:1139–1143.
- Dickhout JG, Hossain GS, Pozza LM, Zhou J, Lhotak S, Austin RC. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2623–2629.
- Vanholder R, Argiles A, Baurmeister U, et al. Uremic toxicity: present state of the art. Int J Artif Organs. 2001;24:695–725.
- Cherla G, Jaimes EA. Role of L-arginine in the pathogenesis and treatment of renal disease. J Nutr. 2004;134:2801S-6.
