Abstract
In this prospective study, we aimed to determine the protective antioxidant role of alpha-lipoic acid (ALA) on development of contrast-induced nephropathy (CIN) in diabetic patients undergoing coronary angiography. Seventy-eight diabetic patients undergoing coronary angiography were included. Thirty-nine patients were randomized to control group and 39 patients to ALA group. Both groups were hydrated on the day of angiography, and the ALA group had also received three doses of “Thioctacid 600 mg HR, MEDA Manufacturing GmbH” in pill form. Serum creatinine clearance, cystatin C, and urinary neutrophil gelatinase-associated lipocalin (NGAL) were studied before and after angiography. We defined CIN as either ≥25% or ≥0.5 mg/dL increase in serum creatinine at 48th hour after angiography. Baseline clinical characteristics were similar in both groups. Mehran risk score and creatinine clearance were comparable in control and therapy groups (5.59 ± 1.96 vs. 5.49 ± 1.73, p = 0.54 and 89 ± 21 vs. 96 ± 24, p = 0.13, respectively). The volumes of contrast media (median values of 80 mL vs. 75 mL) and hydration with saline (2862 ± 447 mL vs. 2637 ± 592 mL) were also similar (p > 0.05). The incidence of CIN was the same (8%) in both the groups. Alterations in serum creatinine, cystatin C, and urinary NGAL levels before and after the procedure were comparable between the ALA and control groups (group p-values were >0.05 in two-way repeated measures analysis of variance). We presented for the first time that ALA therapy added to hydration does not decrease the risk of CIN development in diabetic patients undergoing coronary angiography.
INTRODUCTION
Contrast-induced nephropathy (CIN) is defined as acute impairment of renal functions caused by exposure to contrast media. The main factors for CIN development are patients’ characteristics, type and amount of the contrast agent, and preventive as well as therapeutic approaches applied. In clinical practice, CIN is diagnosed by a relative rise of ≥25% or an absolute rise of 0.5 mg/dL (44 μmol/L) over the baseline serum creatinine level.Citation1–Citation4 Contrast agents are the third most common reason for the hospital-acquired acute renal failures. Today, numerous cardiovascular, peripheral diagnostic and interventional procedures, and tomographic imagings are performed, all of which lead to anticipated diagnoses of CIN in daily practice.Citation5,Citation6
Pathophysiology of the CIN is not explained exactly. Direct toxic effects of the contrast agents on the renal tubular cells, oxidative stress, impairment of renal hemodynamics by increased tubular and renal tissue pressure, and renal medullary hypoxia due to vasoconstriction are the main mechanisms. After the recognition of this important clinical condition, less nephrotoxic agent is being developed and preventive approaches are performed resulting in decreased comorbidities caused by CIN.Citation7,Citation8 The key predisposing factor for CIN development is the pre-existing chronic renal disease. Many risk factors are determined such as diabetes mellitus (DM), congestive heart failure (CHF), volume depletion, advanced age, type and volume of the contrast agent, and exposure to more than one contrast agent in 72 h.Citation9–Citation12 A simple and useful risk scoring system offered by Mehran et al. is most frequently utilized in clinical practice.Citation13
The management of CIN is based on the preventive modalities. Hydration is the best proven approach both for prevention and treatment.Citation10 Many vasoactive agents such as PGE1,Citation14 dopamine,Citation15 fenoldopam,Citation16 theophylline,Citation17 calcium channel blockers,Citation18 and angiotensin converting enzyme inhibitorsCitation19 have been shown to have minimal or no effects on the CIN development. Furthermore, a recently published comprehensive and randomized study revealed debatable results with the use of N-acetylcysteine.Citation20
Alpha-lipoic acid (ALA) is a thiol-containing molecule with antioxidant properties.Citation21 In an animal study, ALA ameliorated histopathologic changes due to renal ischemic reperfusion.Citation22 In a study with end stage renal disease patients on hemodialysis, ALA supplementation reduced high-sensitive C reactive protein (CRP) levels.Citation23 However, there is no clinical study yet to evaluate the possible protective effects of ALA on CIN occurrence. Accordingly, the aim of this single-center prospective randomized trial was to determine the protective antioxidant role of alpha-lipoic acid on the development of CIN in diabetic patients undergoing coronary angiography.
METHODS
Study Population
Seventy-eight consecutive, eligible diabetic patients, to whom elective coronary angiography (CAG) was performed on an outpatient basis in Baskent University Medical School, Ankara Education and Research Hospital between January 2010 and February 2011, were included in the study. There were 31 females and 47 males with a mean age of 65 ± 9 (40–84) years. Thirty-nine patients were randomized to control group and 39 patients to ALA group. ALA group received three doses of “Thioctacid 600 mg HR, MEDA Manufacturing GmbH” in pill form before meal; 30 min before and at 24th and 48th hour of coronary angiography. The study was conducted in accordance with the guidelines proposed in the Helsinki Declaration and was approved by the local ethics committee. Each patient has given informed consent form.
Patients with a serum creatinine of >1.5 mg/dL, known malignancy, liver disease, allergy to contrast media, use of any nephrotoxic agent within 48 h and exposure to contrast agent within 7 days, unstable angina and hemodynamically unstable patients as well as the patients to whom ad-hoc percutaneous coronary intervention (PCI) should be performed were not included. All patients were scanned in terms of risk factors, such as hypertension, diabetes mellitus, smoking, hyperlipidemia, history of coronary artery disease (CAD), and peripheral artery diseases (PAD).
Diagnosis of CIN was made by studying serum creatinine levels just before and at 48th hour of CAG. A relative rise of ≥25% or an absolute rise of 0.5 mg/dL (44 μmol/L) over the baseline serum creatinine level was determined as CIN.Citation1–Citation4 Non-ionic low osmolar contrast agent “iohexol” was used in all patients. The amount of contrast agent used in diagnostic procedures was noted. When a percutaneous coronary intervention was required, it was postponed and performed electively. All patients were hydrated intravenously on the day of CAG; saline infusion was started 3 h before procedure and continued for 24 h with a classical infusion rate of 1 mL/kg/h and the amount of saline was recorded.
Laboratory Measurements
Serum blood urea nitrogen (BUN), creatinine and cystatin C levels, and urinary NGAL levels were studied before CAG. Estimated creatinine clearance was calculated by Cockcroft–Gault formula. During follow-up, measurement of serum creatinine levels was performed at 24th and 48th hours, serum cystatin C levels at 24th hour, and urine NGAL levels at 4th hour of CAG. There was a time interval of 3 h between the last dose of ALA and creatinine level at 48th hour. Blood pressure, amount of hydration, and urine of the patients were recorded.
Serum creatinine level was measured by using Jaffe’s Kinetic Method (Hitachi Modular PP Analyzer, Roche Diagnostics GmbH, Mannheim, Germany). Serum cystatin C level was detected with sandwich enzyme immunoassay method using commercial Human Cystatin C kits (Biovendor Laboratory Medicine, Inc., Modrice, Czech Republic) by DYNEX Technologies DSX Model Microelisa Analyser device (Prague, Czech Republic). Urinary NGAL levels were analyzed with similar method using Human Lipocalin-2/NGAL kits (Biovendor Laboratory Medicine, Inc.).
Statistical Analysis
Continuous variables were presented as mean ± SD if normally distributed, otherwise, as median with 25th and 75th percentiles and interquartile range (IQR). The normality was tested using Shapiro–Wilk test. Levene’s test is used to assess variance homogeneity. Categorical variables were presented as numbers and percentages and were compared using the Fisher’s Exact test. For comparison of the independent continuous variables, t-test or Mann–Whitney U test was used, where appropriate. The laboratory measurements of the same objects in different times were compared using two-way repeated measures analysis of variance including group factor. Before performing this test, a right skewed distribution of the variables was detected. Hence, logarithmic transformation was applied to provide the conditions of this analysis. For multiple post-hoc comparisons, Bonferroni test was utilized. A multivariate logistic regression analysis model was used to determine the predictors of CIN development. The variables included in the model were age, hypertension, treatment with ALA, hydration and contrast medium amounts, baseline levels of creatinine clearance, serum cytidine C, and urinary NGAL. p-Value of <0.05 was considered statistically significant. The SPSS for Windows ver. 17.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for all the statistical calculations.
RESULTS
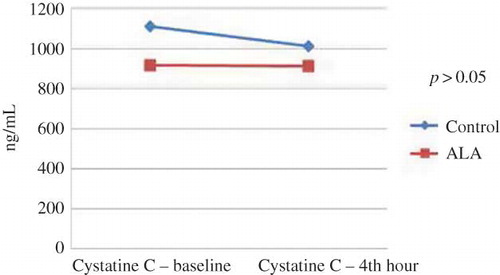
Clinical characteristics and laboratory values of the study patients are given in . There were no differences in terms of age, gender, body mass index, hypertension, and hyperlipidemia (p > 0.05). Baseline creatinine, creatinine clearance, HbA1c, and urine NGAL values were also similar (p > 0.05). However, in control group, baseline cystatin C level was borderline higher than the ALA group (p = 0.04) (). The medications used were comparable except for acetyl salicylic acid and nitrate (). Due to a higher number of CAD histories in ALA group, smoking ratio, ASA, and nitrate use were different between the groups.
Table 1. Baseline clinical parameters, laboratory findings, and medications of the patients.
Table 2. Contrast medium and hydration amounts and CIN incidence between the groups.
Mehran risk scores of the control and ALA groups were 5.59 ± 1.96 and 5.49 ± 1.73 respectively (p = 0.54). Amount of contrast media and hydration with saline were not different (). According to the CIN criteria, three patients in each group (7.7%) developed CIN (p = 1.000) (). In patients diagnosed with CIN, there was no need of prolonged hospitalization or hemodialysis. In these patients, renal functions were ameliorated during follow-up.
Table 3. Comparison of changes in serum cystatin C and urinary NGAL levels between the groups.
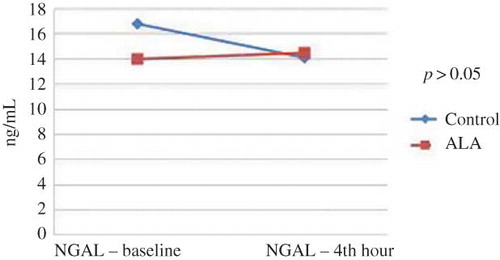
Differences in baseline, 24th hour, and 48th hour levels of blood urea nitrogen (BUN) were not statistically significant (p = 0.59). The changes in serum creatinine levels over time (pre-procedure and at 24th and 48th hours) were significant (p = 0.024). Multiple post-hoc comparisons revealed that this difference was originated by baseline and 48th hour values. However, the alteration in serum creatinine levels did not differ between the ALA and control groups (group p-value = 0.98). When baseline, 24th hour, and 48th hour values of creatinine clearance were compared, there was a significant difference over time (p = 0.011). Multiple post-hoc comparisons showed that this difference was driven by baseline and 48th hour values and 24th hour and 48th hour values. The alteration in creatinine clearance levels did not differ between the ALA and control groups (group p-value = 0.82). There were no significant changes in serum cystatin C levels at baseline and 24th hour after CAG (p = 0.25). It was found that there was no interaction between the group factor and change in cystatin C over time (group p-value = 0.39). Similarly, baseline and 4th hour urine NGAL values were found to be comparable (p = 0.32, group p-value was 0.47; Figures 1 and 2 ).
Multivariate logistic regression analysis revealed baseline levels of serum creatinine clearance (p = 0.01), serum cystatin C (p = 0.009), and amount of contrast medium (p = 0.02) as the independent predictors of CIN occurrence.
DISCUSSION
In the present study, we presented that ALA therapy added to hydration does not decrease the risk of CIN development in diabetic patients undergoing coronary angiography.
In daily practice, numerous cardiovascular and peripheral diagnostic and interventional procedures and tomographic imagings are performed, all of which result in the increased diagnoses of CIN, a kind of acute kidney injury.Citation5,Citation6 Several biomarkers are introduced for early diagnosis of this clinical condition. Plasma cystatin C levels at 24th hour of contrast exposure was shown to determine CIN accurately.Citation24 Supporting this finding, cystatin was an independent predictor of CIN occurrence in our study. Urinary NGAL level measured within 6 h after use of contrast medium was determined as a specific and sensitive marker of CIN.Citation25 Correspondingly, Malyszko et al. declared that NGAL was an early marker and predictor of nephropathy and unstable angina in patients undergoing cardiac catheterization.Citation26 In our study, in each of the control and treatment group, serum creatinine levels were increased and creatinine clearance values were decreased. However, urinary NGAL levels did not change significantly before and at 4th hour of angiography. Similarly, despite a borderline higher basal serum value of the control subjects, changes in cystatin C levels were not significant before and at 24th hour of the procedure. Importantly, alterations in all of these markers did not differ between the groups. In clinical practice, for diagnosis of CIN, certain cut-off values of plasma cystatin C and urinary NGAL levels are not determined. Therefore, CIN incidences calculated from serum creatinine were found to be similar between control and treatment groups. As a result of these findings, we can conclude that treatment with ALA did not influence the development of CIN.
Baseline characteristics such as age, gender, body mass index, creatinine, hemoglobin, HbA1c levels, left ventricular systolic functions, and use of ACE inhibitor/angiotensin II receptor blocker, calcium channel blocker, statin and diuretics were similar between control and treatment groups. The amount of contrast medium as well as the amount of hydration with saline was also comparable. It was essential to check all of these factors to directly observe the possible protective effect of ALA therapy against CIN.
Alpha-lipoic acid (ALA) is a thiol-containing molecule with antioxidant properties. It is found in mitochondria and lysosomes of various tissues.Citation21 Alpha-lipoic acid is rapidly absorbed by the intestine after oral administration, and maximal plasma level is measured after half-hour.Citation21,Citation27 An oral dose of 600 mg daily is successfully used in symptomatic diabetic polyneuropathy.Citation28 In the literature, there are several studies that mention the use of ALA for prevention of acute kidney injury. In the study by Sheila et al., treatment with ALA ameliorated histopathologic findings of ischemia–reperfusion injury of the kidney in rats. This effect was attributed to regulatory functions of ALA in inflammation.Citation22 This favorable result was also confirmed by a recent animal study by Koga et al.Citation29
According to the best of our knowledge, there is no clinical study yet using ALA for the prevention of CIN occurrence in diabetic patients. When physiopathology of CIN is considered, we expected a lower incidence of CIN in ALA group, but we did not derive any beneficial effect. In the study by Jo S.H and colleagues,Citation30 alpha-lipoic acid was given to patients with basal renal insufficiency (CrCl < 60 mL/dk). The authors indicated that additional use of alpha-lipoic acid may be inferior to simple hydration in terms of peak increase of serum creatinine. Additionally, the incidence of CIN was similar in both groups (3.8% in ALA and 3.6% in control group, p = 1.0). The most remarkable finding of this study was a very low rate of incidence of CIN even in patients at high risk. At this point, intensive hydration again seems to be an absolute approach for prevention of CIN.
The small number of study population is the main limiting factor for an adequate number of patients diagnosed with CIN. Additionally, this condition may also explain why changes in urinary NGAL and serum cystatin levels over time were not found to be significant. When our patients were considered in terms of the Mehran risk score,Citation13 in spite of the diabetes mellitus, they were relatively at lower risk for CIN. When patients with higher risk for CIN were included, benefit of the ALA treatment might be pronounced. In accordance with our results, two recent studies defining urinary NGAL as a marker of the early diagnosis of CIN after angiography reported CIN incidences as 8.7% and 10.3%,Citation31,Citation32 respectively. These rates of CIN are similar to that found in our study. The possible explanation for these results is adequate, “in fact”, intensive hydration of the patients. In our study, intensive hydration of the patients with modest Mehran scores had inevitably resulted in a lower incidence of CIN and might have masked beneficial effects of ALA. We measured serum creatinine levels just before and at 24th and 48th hours after CAG. A creatinine follow-up beyond 48 h might result in a higher number of patients being diagnosed with CIN. Meanwhile, a longer monitoring period might also aid in observing positive results. Although we aimed to evaluate the acute effects of ALA therapy on CIN occurrence, after long-term use of ALA, CIN ratio might also be diminished.
In conclusion, we demonstrated that ALA therapy added to hydration does not decrease the risk of CIN development in diabetic patients undergoing coronary angiography. There is a great lack of clinical studies regarding protective and therapeutic effect of ALA on the contrast nephropathy, at this point; despite the limitations mentioned above, our study could be an inspiration to further trials.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Davidson C, Stacul F, McCullough PA, CIN consensus working panel. Contrast medium use. Am J Cardiol. 2006;98:42K–52K.
- McCullough PA, Adam A, Becker CR, CIN consensus working panel. Epidemiology and prognostic implications of contrast-induced nephropathy. Am J Cardiol. 2006;98:5K–13K.
- Briguori C, Airoldi F, Andrea DD, Renal insufficiency following contrast media administration trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–1217.
- McCullough P. Outcomes of contrast-induced nephropathy: experience in patients undergoing cardiovascular intervention. Catheter Cardiovasc Interv. 2006;67:335–343.
- Cronin RE. Contrast-induced nephropathy: pathogenesis and prevention. Pediatr Nephrol. 2010;25:191–204.
- Pannu N, Wiebe N, Math M, Stat P, Tonelli M. Prophylaxis strategies for contrast-induced nephropathy. J Am Med Assoc. 2006;295:2765–2779.
- Persson PB, Tepel M. Contrast medium-induced nephropathy: the pathophysiology. Kidney Int. 2006;69:8–10.
- Goldenberg I, Matetzky S. Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies. CMAJ. 2005;172:1461–1471.
- Lindholt JS. Radiocontrast induced nephropathy. Eur J Vasc Endovasc Surg. 2003;25:296–304.
- Stacul F, Adam A, Becker CR, CIN consensus working panel. Strategies to reduce the risk of contrast induced nephropathy. Am J Cardiol. 2006;98:59–77.
- Gruberg L, Mintz GS, Mehran R, The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542–1548.
- Rihal CS, Textor SC, Grill DE, Incidence and prognostic importance of renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264.
- Mehran R, Aymong ED, Nikolsky E, A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399.
- Sketch MH Jr, Whelton A, Schollmayer E, Prostaglandin E1 Study Group. Prevention of contrast media-induced renal dysfunction with prostaglandin E1: a randomized, double-blind, placebo-controlled study. Am J Ther. 2011;8:155–162.
- Gare M, Haviv YS, Ben-Yehuda A, The renal effect of low-dose dopamine in high-risk patients undergoing coronary angiography. J Am Coll Cardiol. 1999;34:1682–1688.
- Stone GW, McCullough PA, Tumlin JA, CONTRAST investigators. Fenoldopam mesylate for the prevention of contrast-induced nephropathy: a randomized controlled trial. J Am Med Assoc. 2003;290:2284–2291.
- Matejka J, Varvarovsky I, Vojtisek P, Prevention of contrast-induced acute kidney injury by theophylline in elderly patients with chronic kidney disease. Heart Vessels. 2010;25:536–542.
- Khoury Z, Schlicht JR, Como J, The effect of prophylactic nifedipine on renal function in patients administered contrast media. Pharmacotherapy. 1995;15:59–65.
- Patel K, King CA, Jovin IS. Angiotensin-converting enzyme inhibitors and their effects on contrast-induced nephropathy after cardiac catheterization or percutaneous coronary intervention. Cardiovasc Revasc Med. 2011;12:90–93.
- ACT Investigators. Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized Acetylcysteine for Contrast-induced nephropathy Trial (ACT). Circulation. 2011;124:1250–1259.
- Cakatay U. Pro-oxidant actions of α-lipoic acid and dihydrolipoic acid. Med Hypotheses. 2006;66:110–117.
- Sehirli O, Sener E, Cetinel S, Yuksel M, Gedik M, Sener G. A-lipoic acid protects against renal ischemia-reperfusion injury in rats. Clin Exp Pharmacol Physiol. 2008;35:249–255.
- Khabbazi T, Mahdavi R, Safa J, Pour-Abdollahi P. Effects of alpha-lipoic acid supplementation on inflammation, oxidative stress, and serum lipid profile levels in patients with end-stage renal disease on hemodialysis. J Ren Nutr. 2012;22:244–250.
- Briguori C, Visconti G, Rivera NV, Cystatin C and contrast-induced acute kidney injury. Circulation. 2010;121:2117–2122.
- Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024.
- Malyszko J, Bachorzewska-Gajewska H, Poniatowski B, Urinary and serum biomarkers after cardiac catheterization in diabetic patients with stable angina and without severe chronic kidney disease. Ren Fail. 2009;31:910–919.
- Singh U, Jialal I. Alpha lipoic acid supplementation and diabetes. Nutr Rev. 2008;66:646–657.
- McIlduff CE, Rutkove SB. Critical appraisal of the use of alpha lipoic acid (thioctic acid) in the treatment of symptomatic diabetic polyneuropathy. Ther Clin Risk Manag. 2011;7:377–385.
- Koga H, Hagiwara S, Kusaka J, New α-lipoic acid derivative, DHL-HisZn, ameliorates renal ischemia-reperfusion injury in rats. J Surg Res. 2012;174:352–358.
- Jo SH, Kim HS, Lee JH, Evaluation of alpha lipoic acid to prevent contrast induced nepphropathy: A prospective randomised trial. In: Angioplasty Summit Abstracts, E-Poster, Fourteenth Annual Symposium: Angioplasty Summit 2009 TCT Asia Pacific, April 22–29, 2009.
- Ling W, Zhaohui N, Ben H, Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract. 2008;108:c176–c181.
- Li W, Li D, Xu T, Zhang Y, Zhu H, Han F. The value of urinary NGAL on the early diagnosis of contrast induced nephropathy in patients undergoing percutaneous coronary intervention. Heart. 2011;97:A150.