Abstract
Background: Decreased tryptophan (TRP) and increased kynurenine (KYN) and kynurenic acid (KYNA) in blood have been reported in patients and experimental animals with renal diseases. We investigated if these compounds could be used as new biomarkers for the assessment of renal function. Methods: Eighty hospitalized hypertensive patients (20 with chronic kidney disease (CKD), and other 60 were considered as control) were enrolled for the investigation. Plasma TRP, KYN, and KYNA were determined by high-performance liquid chromatography. Change rate (CR) was employed to evaluate the sensitivity of the parameters of renal function. Results: CR of plasma KYNA/TRP ratio (+103%) was much higher than the CRs of blood urea nitrogen (+44%), serum creatinine (+56%) and estimated glomerular filtration rate (–35%). Plasma KYNA/TRP ratio was in close relationship with blood urea nitrogen (r = 0.622), serum creatinine (r = 0.797), urine micro-albumin/24-h (r = 0.518) and estimated glomerular filtration rate (r = –0.662), respectively, with all p-values <0.001. Conclusions: Plasma KYNA/TRP ratio was sensitive and reliable to indicate renal function and could be used as a new biomarker to assess the risk or presence of kidney disease.
INTRODUCTION
Renal function, in nephrology, is an indication of the state of the kidney and its role in renal physiology. Basic renal function includes excretion of wastes, acid-base homeostasis, osmolality regulation, blood pressure regulation, and hormone secretion. Chronic kidney disease (CKD) is a progressive loss in renal function over a period of months or years. Thereafter, wastes produced from biochemical metabolism will be accumulated, and homeostasis of acid-base will be broken down etc. As a result, uratemic syndrome, metabolic syndrome, and endothelial dysfunction will occur during the process of CKD. Clinically, doctors use blood levels of the waste substances to determine renal function, such as blood urea nitrogen (BUN),Citation1 uric acid,Citation1,Citation2 and serum creatinine (SCr),1 which is normally used to calculate estimated glomerular filtration rate (eGFR).Citation3 Blood concentrations of some other compounds were also reported to have correlation with CKD, such as cystatin C.Citation4 Measurement of substances in urine is another way to determine whether a patient is suffering from kidney disease. The most common one is urine micro-albumin (UmAlb).Citation5
Kynurenine (KYN) and kynurenic acid (KYNA) are the metabolites of tryptophan (TRP) which is an essential amino acid and plays an important role in protein synthesis. Accumulating evidences show that blood level of TRP was decreased while KYN and KYNA were increased in patients with CKD.Citation6–Citation8 This phenomenon has been linked to the increased activity of the enzymes catalyzing the reactions in TRP–KYN metabolism pathway, including indoleamine 2,3-dioxygenase (IDO, EC 1.13.11.52),Citation9 tryptophan 2,3-dioxygenase (TDO, EC 1.13.11.11)Citation7,Citation10 and kynurenine aminotransferase (KAT, EC 2.6.1.7).10
The present study measured the plasma concentrations of TRP, KYN, and KYNA by high-performance liquid chromatography (HPLC) in hypertensive patients with and without CKD. Change rate (CR) was used to evaluate the sensitivity of the parameters for measuring renal function. The relationship between renal function and plasma concentrations of TRP, KYN, and KYNA, and their ratios, KYN/TRP, KYNA/KYN, and KYNA/TRP, were also examined.
MATERIALS AND METHODS
Study Population
Eighty hypertensive patients hospitalized in the Hypertension Ward, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, were enrolled for the investigation. Twenty (n = 3, 5, 9, and 3 for CKD stage 1, 2, 3, and 4, respectively) of them (age 58 ± 15 years, 15 males) were diagnosed with CKD according to the National Kidney Foundation (NKF) criteria.Citation11 The other sixty (age 53 ± 13 years, 45 males) were considered as control for a fair comparison because plasma levels of TRP and KYN were a little bit different between healthy subjects and patients with essential hypertension.Citation12 Ethical approval has been obtained from the ethics committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. A written informed consent for biochemical analyses was obtained from all participants.
Blood Sampling
Blood was drawn by experienced phlebotomists in the morning after overnight fasting. Venous blood samples were collected in Vacutainer tubes containing lithium heparin as anticoagulant (BD Vacutainer®, USA) and centrifuged at 4000 rpm for 5 min. Plasma was separated and kept frozen at −20˚C until assayed.
Analysis of Plasma TRP, KYN, and KYNA
Plasma TRP, KYN, and KYNA were determined simultaneously using a developed standard-addition HPLC method reported previously.Citation13 An Agilent 1100 series LC system (Agilent Technologies, Germany) with a variable wavelength detector at wavelength 365 nm and a fluorescence detector at wavelengths excitation 344 nm and emission 398 nm were used for the analysis. An Agilent HC-C18 (2) column was employed as an analytical column (250 × 4.6 mm i.d.; 5 µm particle size). Mobile phase was composed of 20 mmol/L NaAc, 3 mmol/L ZnAc2, and 7% acetonitrile without any extra pH adjustment. Separation was achieved at an ambient temperature with flow rate 1 mL/min. Concentrations were calculated from peak areas. An HP Chemstation software was employed to control HPLC system and process chromatographic data.
Assessment of Renal Function
Renal function was assessed using the following indices: BUN, SCr, UmAlb/24-h, and eGFR. BUN, SCr, and UmAlb/24-h were measured by the Department of Laboratory Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China. eGFR was calculated by the modification of diet in renal disease (MDRD) formula.
Statistics
Data were analyzed as mean ± SD and compared using Student’s t-test. GraghPad Prism software was used to calculate correlation coefficient using linear regression analysis. Significance was determined as p < 0.05.
RESULTS
Renal Function and Plasma TRP, KYN, and KYNA
The clinical parameters of renal function and plasma TRP, KYN, and KYNA are summarized in . Control and CKD groups had a same gender ratio (male/female = 3) and similar age composition (p = 0.180). As expected, the mean levels of BUN, SCr, and UmAlb/24-h were significantly higher whereas eGFR was significantly lower in CKD than in control (all p < 0.001). Compared with control, CKD had significantly lower level of TRP (p = 0.017) together with significantly higher levels of KYN and KYNA (both p < 0.001), resulting in significantly higher KYN/TRP and KYNA/TRP ratios (both p < 0.001). KYNA/KYN ratio was significantly higher in CKD than in control (p < 0.001) due to a much higher elevation of KYNA. To evaluate the sensitivity of the parameters of renal function, we calculated the CR by using the formula CR (%) = (CKD – Control)/Control × 100. shows that CR of eGFR and TRP changed in negative direction and, CR of others changed in positive direction. CR of the ratio was much higher than that of the correlative compound [KYN/TRP (+44%) > KYN (+27%); KYNA/TRP (+103%) > KYNA (+79%)]. CR of KYNA/TRP was the highest one among all of the observed parameters.
Table 1. Renal function and plasma TRP, KYN, and KYNA (mean ± SD).
Correlation Between Renal Function and Plasma TRP, KYN, and KYNA
Next we examined the correlation between renal function and plasma TRP, KYN, and KYNA. shows that TRP was negatively correlated with BUN (p < 0.05) and UmAlb/24-h (p < 0.01); and not correlated with SCr and eGFR. KYN, KYNA, KYN/TRP, KYNA/KYN, and KYNA/TRP were positively correlated with BUN, SCr, and UmAlb/24-h (all p < 0.001 except < 0.01 for the correlation between KYN and UmAlb/24-h); and negatively correlated with eGFR (all p < 0.001). Among them, KYNA/TRP showed the best correlation with the parameters of renal function (mean correlation coefficient = 0.650).
Table 2. Correlations between parameters of renal function and plasma TRP, KYN, and KYNA (n = 80).
Relationship Between KYN and TRP or KYNA
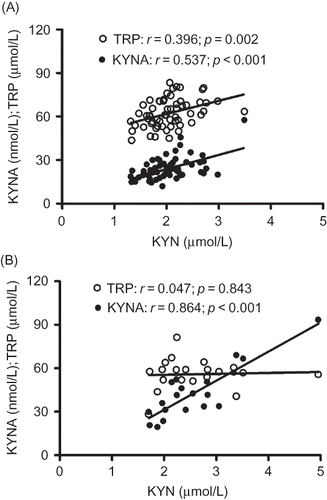
The relationship between substrate and product could represent the activity of an enzyme. In biochemistry, people like to use the ratios of KYN/TRP and KYNA/KYN as the evidences for the activities of IDO9 and KAT.Citation14 Under normal circumstances, higher concentration of substrate will produce more products. Here KYN is an intermediate between TRP and KYNA. On the one hand, KYN is the product of TRP and, on the other hand, it is the substrate of KYNA. shows the correlation between KYN and TRP or KYNA (A, control; B, CKD). KYN was correlated with KYNA in both control and CKD (both p < 0.001); but it was correlated with TRP only in control (p = 0.002) and not in CKD (p = 0.843).
DISCUSSION
Decreased TRP and increased KYN and KYNA in blood have been reported in patients and experimental animals with renal disease.Citation7,Citation9,Citation10,Citation15 In this report, our data were in agreement with those published previously. We found that plasma KYNA/TRP ratio had the highest CR (+103%) among all of the observed parameters for measuring renal function due to the significant up-regulation of KYNA (+79%) and down-regulation of TRP (–9%) in CKD. Ratio contains two determinants. One is the numerator and the other is the denominator. The CR of the ratio is up to the change direction of these two determinants. When numerator changes in up-regulation and denominator in down-regulation under certain circumstance, the CR of the ratio will be bigger than the CR of either numerator or denominator. In other words, the ratio enlarges the changes by using two determinants changing in opposite direction. The highest CR of KYNA/TRP suggests that it may be more sensitive than traditional clinical parameters to reflect the changes of renal function. TRP showed only a minimal CR (–9%), and its p-value was not as small as that of KYN and KYNA (). Besides, plasma TRP showed only poor correlations with BUN and UmAlb/24-h, and no correlations with SCr and eGFR (). One reason is that TRP exists in equilibrium between a free and albumin-bound pool in blood resulting in a less change of concentration. The other possible reason may be the reabsorption of amino acids. Many of the CKD patients participated in this investigation belonged to early stage, and the kidneys still kept some basic functions well.
Our results demonstrated that plasma concentrations of KYN and KYNA and the ratios of KYN/TRP, KYNA/KYN, and KYNA/TRP were correlated with the parameters of renal function, including BUN, SCr, UmAlb/24-h, and eGFR. Mean correlation coefficient of KYN/TRP was higher than that of KYN, and KYNA/TRP than that of KYNA, suggesting that renal function had a better correlation with the ratio than the correlative compound ().
As the main route of eliminating KYN is renal excretion,Citation16 we have right to believe that the excretory impairment of kidney should be responsible for the elevation of blood KYN in CKD patients. However, increased KYN was found not only in blood of CKD patients but also in urine of the experimental animals with renal insufficiency.Citation7 Therefore, the elevation of blood KYN may be attributable not only to the excretory impairment of kidney but also to the up-regulation of the enzymes related to the synthesis of KYN, such as IDO.Citation17,Citation18
Normally, concentration of product has a positive correlation with its substrate. KYN is an intermediate between TRP and KYNA, and its concentration should be positively correlated with TRP and KYNA in normal condition. As shown in , KYN was correlated with KYNA in both control and CKD; but correlated with TRP only in control, not in CKD. This result further demonstrated that IDO and/or TDO were involved in CKD because reduced TRP would have been expected to decrease, not increase, KYN concentration in blood.
IDO and TDO are the isoenzymes. They catalyze the same chemical reaction for the conversion of TRP to N-formylkynurenine, which is then transformed to KYN by kynurenine formylase. IDO is an immunomodulatory enzyme produced by some alternatively activated macrophages and other immunoregulatory cells. It is up-regulated by certain cytokines and inflammatory molecules. In CKD, the elevated plasma KYN/TRP ratio was linked to the increased activation of the immune response.Citation9 TDO is assumed to be normally restricted to the liver in mammals.Citation19 Studies performed by Saito et al.7 indicated that chronic renal insufficiency led to increased activity of TDO, while IDO seemed to play only a minor role. Pawlak et al.10 demonstrated that chronic renal failure was associated with increased activity of TDO in the liver, not IDO in extrahepatic tissues including kidney. It still remains unknown why kidney disease triggers the activation of TDO in liver.
KAT is an aminotransferase which participates in TRP metabolism to catalyze the synthesis of KYNA, an endogenous antagonist at the glycine site of the N-methyl-d-aspartate (NMDA) as well as at the alpha 7 nicotinic cholinergic receptors. KYNA produced from periphery excretes through tubular secretion. In healthy individuals, the concentration of urinary KYNA is approximately 200 times as high as that of plasma KYNA concentration.13,20 The proposed mechanism of the increased plasma KYNA in renal failure preferred the impaired excretion to the enhanced biosynthesis based on the observed inhibition of KAT activity in kidney homogenate by KYNA.10 It was in the same literature, the authors found much higher KAT activity and KYNA concentration in kidneys of the experimental rats with chronic renal failure. We believe that enzymatic conversion of KYN to KYNA by KAT could not be excluded, for KAT exists abundantly in kidney21 and could be activated during the process of CKD. Besides, the increased blood KYN provides more substrate for the synthesis of KYNA. The better explanation may be that both the impaired excretion and increased activities of the enzymes including KAT, TDO and/or IDO were involved resulting in the elevation of KYNA in blood.
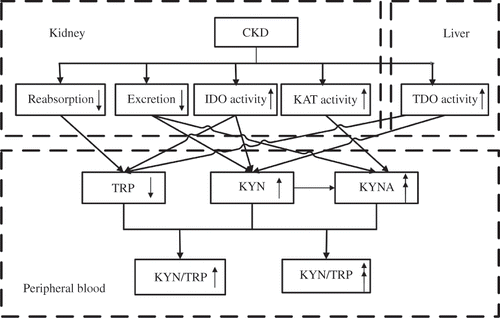
is a schematic diagram which summarizes the changes of blood TRP, KYN, KYNA, KYN/TRP, and KYNA/TRP in CKD patients.
CONCLUSION
Decreased TRP and increased KYN and KYNA in plasma of CKD patients indicate the attenuated excretion of the kidneys and the increased activities of some relative enzymes, such as TDO, IDO, and KAT. Plasma KYNA/TRP ratio is sensitive and reliable to indicate renal function and could be utilized as a new biomarker for the diagnosis of kidney disease, as well as its severity.
ACKNOWLEDGMENTS
The author wishes to express his appreciation to Dr. Yan Wang for scientific discussions and to Dr. Bingxin Xu for providing plasma samples. This work was supported by the research grant from the Shanghai Municipal Health Bureau (2009206).
Declaration of interest
The author reports no conflicts of interest. The author alone is responsible for the content and writing of this article.
REFERENCES
- Liu BC, Wu XC, Wang YL, Investigation of the prevalence of CKD in 13,383 Chinese hospitalized adult patients. Clin Chim Acta. 2008;387:128–132.
- Filiopoulos V, Hadjiyannakos D, Vlassopoulos D. New insights into uric acid effects on the progression and prognosis of chronic kidney disease. Ren Fail. 2012;34:510–520.
- Cai F, Ren L, Liu H, The relationship between the level of serum creatinine, modified diet and renal disease formula, Cockcroft-Gault formula, and renal tubulointerstitial lesion. Ren Fail. 2012;34:334–337.
- Robles NR, Mena C, Cidoncha J. Estimated glomerular filtration rate from serum cystatin C: significant differences among several equations results. Ren Fail. 2012;34:871–875.
- Warnock DG, Muntner P, McCullough PA, Kidney function, albuminuria, and all-cause mortality in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. Am J Kidney Dis. 2010;56:861–871.
- Pawlak K, Kowalewska A, Mysliwiec M, Pawlak D. Kynurenine and its metabolites—kynurenic acid and anthranilic acid are associated with soluble endothelial adhesion molecules and oxidative status in patients with chronic kidney disease. Am J Med Sci. 2009;338:293–300.
- Saito K, Fujigaki S, Heyes MP, Mechanism of increases in L-kynurenine and quinolinic acid in renal insufficiency. Am J Physiol Renal Physiol. 2000;279:F565–F572.
- Pawlak K, Brzosko S, Mysliwiec M, Pawlak D. Kynurenine, quinolinic acid—the new factors linked to carotid atherosclerosis in patients with end-stage renal disease. Atherosclerosis. 2009;204:561–566.
- Schefold JC, Zeden JP, Fotopoulou C, Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uratemic symptoms. Nephrol Dial Transplant. 2009;24:1901–1908.
- Pawlak D, Tankiewicz A, Matys T, Buczko W. Peripheral distribution of kynurenine metabolites and activity of kynurenine pathway enzymes in renal failure. J Physiol Pharmacol. 2003;54:175–189.
- K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266.
- Xu B, Zhan Y, Gao P, Zhu D, Zhao J. Changes of plasma concentrations of tryptophan, kynurenine and kynurenic acid in essential hypertensive patients. Lab Med. 2012;27:624–627 (Chinese).
- Zhao J, Gao P, Zhu D. Optimization of Zn2+-containing mobile phase for simultaneous determination of kynurenine, kynurenic acid and tryptophan in human plasma by high performance liquid chromatography. J Chromatogr B. 2010;878:603–608.
- Stoy N, Mackay GM, Forrest CM, Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J Neurochem. 2005;93:611–623.
- Pawlak D, Tankiewicz A, Buczko W. Kynurenine and its metabolites in the rat with experimental renal insufficiency. J Physiol Pharmacol. 2001;52:755–766.
- Holmes EW. Determination of serum kynurenine and hepatic tryptophan dioxygenase activity by high-performance liquid chromatography. Anal Biochem. 1988;172:518–525.
- Eleftheriadis T, Yiannaki E, Antoniadi G, Plasma indoleamine 2,3-dioxygenase and arginase type I may contribute to decreased blood T-cell count in hemodialysis patients. Ren Fail. 2012;34:1118–1122.
- Eleftheriadis T, Antoniadi G, Liakopoulos V, Stefanidis I, Galaktidou G. Plasma indoleamine 2,3-dioxygenase concentration is increased in hemodialysis patients and may contribute to the pathogenesis of coronary heart disease. Ren Fail. 2012;34:68–72.
- Knox WE, Auerbach VH. The hormonal control of tryptophan peroxidase in the rat. J Biol Chem. 1955;214:307–313.
- Zhao J, Chen H, Ni P, Simultaneous determination of urinary tryptophan, tryptophan-related metabolites and creatinine by high performance liquid chromatography with ultraviolet and fluorimetric detection. J Chromatogr B. 2011;879:2720–2725.
- Mason M. Kynurenine transaminase of rat kidney; a study of coenzyme dissociation. J Biol Chem. 1957;227:61–68.