Abstract
Objective: Although colchicine is effective on prevention and regression of amyloidosis in many cases, rate of unresponsiveness to colchicine therapy is not too low. However, there is no sufficient data about which factors effect to response of colchicine therapy on regression of amyloidosis. Materials and methods: 24 patients with renal amyloidosis were enrolled into the study. The patients were divided in two groups according to urinary protein excretions: non-nephrotic stage (14/24) and nephrotic stage (10/24). The patients were also categorized according to the etiology of amyloidosis; familial Mediterranean fever (FMF)-associated amyloidosis (15/24) versus rheumatoid disorders (RD)-associated amyloidosis (9/24). The changes of amount of proteinuria and estimated glomerular filtration rates were investigated after colchicine treatment started in these groups. Results: The mean follow-up period was 27.7 ± 19.2 months. After initiating colchicine therapy, the degree of proteinuria was decreased higher than 50% in 11/14 (78%) of non-nephrotic patients and elevated only in three (22%) patients. In nephrotic group, proteinuria was increased in 5/10 (50%) of patients. Glomerular filtration rates were stable in nephrotic and non-nephrotic groups. Presenting with nephrotic syndrome was higher in RD-associated amyloidosis (RD_A) group (5/9) than FMF-associated amyloidosis (FMF_A) group (5/15) without statistical significance (p > 0.05). After colchicine treatment, proteinuria was decreased in 12/15 patients in FMF_A group, however, the significant decreasing of proteinuria was not observed in RD_A group (p = 0.05 vs. p > 0.05). Conclusion: Colchicine therapy was found more effective in low proteinuric stage of amyloidosis. The beneficial effect of colchicine therapy was not observed in patients with RD- associated amyloidosis.
Introduction
Amyloidosis is the most severe complication of chronic inflammatory diseases such as familial Mediterranean fever (FMF) and other rheumatologic disorders (RD). Renal amyloidosis presents itself with persistent, progressive proteinuria, leading to nephrotic syndrome and progressive nephropathy leading to end-stage renal disease (ESRD).Citation1–3 Colchicine is the most important treatment option in amyloidosis and daily use of colchicine can prevent development of amyloidosis especially in FMF patients.Citation1,Citation3 There are few anecdotal reports about improvement of proteinuria with colchicine treatment in the course of renal amyloidosis; however, there is no sufficient data about long-term response of proteinuria and renal functions to colchicine treatment in patients with amyloidosis except FMF.Citation4–6 In this study, we aimed to evaluate the effect of colchicine treatment in renal amyloidosis patients due to various etiologies and clinical presentations.
Materials and methods
The study was approved by the local ethics committee and conducted in accordance with the ethical principles described by the Declaration of Helsinki. Patients with secondary amyloidosis enrolled into the study. AA amyloidosis was diagnosed by kongo-red staining of kidney biopsy. Between the years 2002 and 2010, totally 37 patients with AA amyloidosis were diagnosed. Thirteen patients were excluded because of short clinical course (i.e., less than 3 months), insufficient data or interrupted colchicine treatment. Twenty-four (15 male, 9 female) patients with renal amyloidosis were enrolled into the study. All patients were treated with colchicine 1–2 mg/day after the diagnosis of amyloidosis. Clinical characteristics, laboratory findings and outcomes of patients were noted. Daily urinary protein excretions were calculated, and estimated glomerular filtration rates (eGFR) were calculated using the modification of diet in renal disease (MDRD) formula.Citation7 The patients were divided into two groups according to their renal protein excretions: non-nephrotic stage; proteinuria <3.5 g/1.73 m2 per day and nephrotic stage, proteinuria ≥3.5 g/1.73 m2 per day. The patients were also categorized based on the etiology of amyloidosis (FMF-associated amyloidosis vs. RD-associated amyloidosis).
All statistical analyses were performed using the SPSS for Windows, version 15.0 (Chicago, IL). Unless otherwise stated, results were expressed as means ± standard deviation. p Value < 0.05 was considered statistically significant.
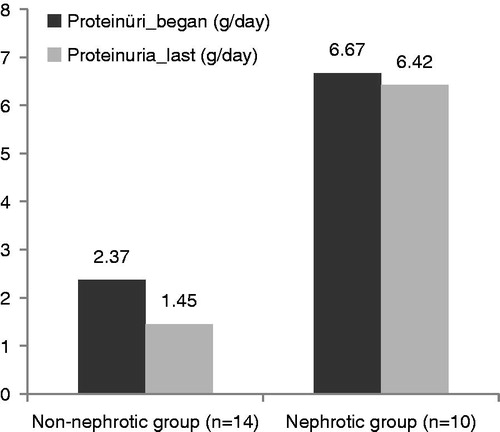
Results
Twenty-four patients (15 male, 9 female) were enrolled into the study. AA amyloidosis were related with FMF in 15/24 (62.5%) patients and 9/24 (37.5%) patients were related with other rheumatologic disorders [ankylosing spondylitis in 4/24 (16.7%) patients, rheumatoid arthritis in 2/24 (8.3%) patients and Behcet’s disease in 3/24 (12.5%) patients]. The mean age of the patients was 40 ± 12.8 years (range 22–71 years). The mean follow-up period was 27.7 ± 19.2 months (range 4–64 months). At presentation, 14 patients were at the non-nephrotic proteinuric stage, 10 patients were at the nephrotic stage. Nine patients had normal eGFR at presentation and the eGFR was lower than 80 mL/min/1.73 m2 in 15 patients. After initiating colchicine therapy, the degree of proteinuria was decreased more than 50% in 11/14 (78%) of non-nephrotic patients and elevated only in three (22%) patients. In the nephrotic group, proteinuria was increased in 5/10 (50%) of patients. Complete resolution of proteinuria (<200 mg/day) was observed only in 3/14 patients in non-nephrotic stage. eGFR levels were stable in nephrotic and non-nephrotic groups. One patient with nephrotic syndrome started to hemodialysis. Proteinuria was higher in the nephrotic group (6.67 ± 4.24 vs. 2.37 ± 1.78; p < 0.005). The eGFR and albumin levels were higher in the non-nephrotic proteinuria group without any statistical significance and other laboratory findings were statistically similar in both groups (). The amount of proteinuria was compared before and after colchicine therapy. Proteinuria decreased from 2.37 ± 1.78 g/day to 1.45 ± 1.17 g/day (p < 0.05) in the non-nephrotic group and from 6.67 ± 4.24 g/day to 6.42 ± 2.56 g/day (p > 0.05) in the nephrotic group (). The mean level of serum albumin increased from 3.6 ± 0.63 g/dL to 3.8 ± 0.52 g/dL in the non-nephrotic group (p = 0.07) and increased from 3.28 ± 0.65 g/dL to 3.37 ± 0.66 g/dL in the nephrotic group (p > 0.05). eGFR increased from 95.5 ± 4.6 mL/min to 97.9 ± 48.5 mL/min in the non-nephrotic group (p > 0.05) and increased from 68.5 ± 46 to 70.3 ± 47.1 mL/min in the nephrotic group without any statistical significance (p > 0.05) ().
Table 1. Comparison of clinical and laboratory findings of non-nephrotic and nephrotic proteinuria groups.
Table 2. Comparison of laboratory findings of patients before and after the colchicine treatment.
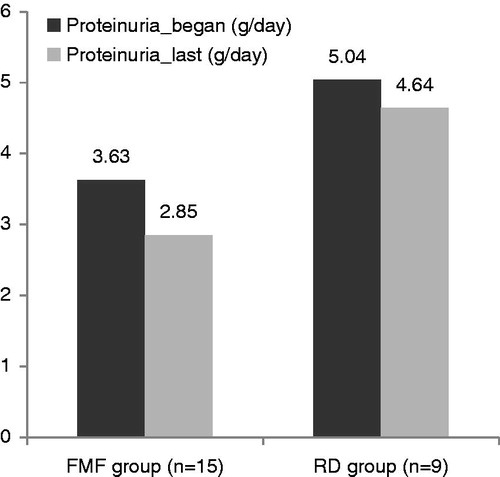
In the subgroup analysis, the patients were classified according to FMF-associated amyloidosis (FMF_A) (n = 15) and to rheumatologic disorders-associated amyloidosis (RD_A) (n = 9) (). Nephrotic syndrome at presentation was slightly higher in the RD_A group (5/9) than the FMF_A group (5/15) without statistical significance (p > 0.05). The FMF_A group (35.2 ± 9.8 years) was younger than the RD_A group (48 ± 13.6 years) (p = 0.02). After colchicine treatment proteinuria was decreased in 12/15 patients in the FMF_A group, however, a significant decrease in proteinuria was not observed in the RD_A group (p = 0.05 vs. p > 0.05). Additionally, the proteinuria decrease rate was significantly higher in the FMF_A group (21.5%) than the RD_A group (8.6 %) (p < 0.05). The mean eGFR level at the presentation was lower (57.6 ± 40.7 mL/min) the in RD_A group than the FMF_A group (82.5 ± 36.5) (p < 0.05) and these values did not change significantly after therapy (52.6 ± 37 vs. 80 ± 33.3) (p < 0.05).
Table 3. Comparison of laboratory findings of FMF-associated and RD-associated amyloidosis groups.
Presenting with nephrotic syndrome was higher in the RD_A group (5/9) than the FMF_A group (5/15) without a statistical significance (p > 0.05). The FMF_A group (35.2 ± 9.8 years) was younger than the RD_A group (48 ± 13.6 years) (p = 0.02). After colchicine treatment proteinuria was decreased in 12/15 patients in the FMF_A group, however, a significant decrease in proteinuria was not observed in the RD_A group (p < 0.05 vs. p > 0.05). Additionally, after initiating colchicine therapy, the proteinuria decrease rate was significantly higher in the FMF_A group (21.5%) than the RD_A group (8.6%) (p < 0.05) (). The mean eGFR at the presentation was lower (57.6 ± 40.7 mL/min) in the RD_A group than the FMF_A group (82.5 ± 36.5) (p < 0.05) and these values did not change significantly after the therapy (52.6 ± 37 vs. 80 ± 33.3).
Discussion
In this study, we observed that the amount of proteinuria was significantly decreased after colchicine therapy in renal amyloidosis patients with non-nephrotic range proteinuria. Glomerular filtration rates were not changed during the follow-up period in both nephrotic and non-nephrotic proteinuria groups under colchicine treatment. The colchicine treatment seems to be more effective in decreasing the proteinuria levels at early stages of amyloidosis. Additionally, beneficial effect of the treatment was observed in both groups regarding stabilization of glomerular filtration rates. In the subgroup analysis, proteinuria levels were higher and eGFR were lower in patients with rheumatologic disease-associated amyloidosis those in FMF-associated amyloidosis patients. The patients with FMF-associated amyloidosis responded to the colchicine treatment, however, similar response rate was not observed in patients with RD-associated amyloidosis patients.
In the cases with FMF, the proteinuria development risk was defined at approximately 2% of patients.Citation4,Citation8 With a presence of amyloidosis, approximately 20% percent of patients had nephrotic range proteinuria and renal functions were deteriorated in all of these patients with nephrotic syndrome.Citation4 In patients who have non-nephrotic proteinuria, improvement of proteinuria reported approximately in 6% and stabilization in 79%.Citation4 In our study, decreasing of proteinuria higher than 50% was defined approximately in 80% of patients with non-nephrotic proteinuria. Our study revealed that, colchicine treatment reduced proteinuria in half of the patients with nephrotic syndrome. Many studies emphasized that, early initiation and absolute compliance with colchicine therapy are the most important approaches for regression in preservation of proteinuria in FMF-associated amyloidosis patients.Citation4,Citation8–12 The most beneficial effect of early initiation of colchicine therapy on decreasing proteinuria in pediatric amyloidosis patients is also consistent with these resultsCitation2,Citation13 Additionally, our study revealed that, response to colchicine treatment should also depend on the etiology of amyloidosis. In the subgroup analysis, the amyloidosis was related with FMF in all of the responder to colchicine treatment. RD-associated amyloidosis was most frequently presented with nephrotic syndrome and in our study, all patients with RD-associated amyloidosis were resistant to colchicine therapy.
Amyloidosis is presented with nephrotic syndrome and impaired kidney functions approximately in half of the patients with rheumatologic disorders and the presence of amyloidosis is a very poor prognostic factor for these patients.Citation14,Citation15 In our study, RD-associated amyloidosis with nephrotic syndrome and impaired kidney functions were observed in 55% of the participants and the results were consistent with the previous data.
The most important factor is severity and length of inflammation period for deposition of amyloidosis. The development of amyloidosis is characterized by a pre-deposition period during inflammation with induced elevation of circulating SAA levels. The second phase is the deposition of amyloid fibrils in the tissues.Citation16,Citation17 Amyloidosis is a reversible process if the reduction of amyloid fibrils has been achieved by discontinuing of inflammation.Citation18–20 The previous experimental studies revealed that, amyloid clearance period from tissues continued for approximately six months.Citation18,Citation20 We can shortly summarize that, occurrence of amyloidosis is related with stimulation of the production of serum A amyloid and deterioration of production/clearance ratio due to prolonged inflammation.
FMF is generally characterized with attacks and the severe inflammation is observed only during the attack period and they can be controlled with colchicine treatment.Citation1 However, other rheumatologic disorders are characterized with continuing severe inflammation and therefore clinicians need potent immunosuppressant drugs or bioactive agents to control the disease activity and inflammation-induced amyloidosis.Citation21–24 Probably, low efficiency of colchicine was inadequate in controlling inflammation and accumulation of AA amyloid in patients with RD.
Previous studies reported that therapeutic effect of colchicine in amyloidosis depends mainly on two factors; the first factor is the serum creatinine and eGFR at first presentation and the second factor is the drug dose and compliance of the therapy.Citation2,Citation8,Citation12 However, there is no sufficient data about the relation between etiology of amyloidosis and colchicine therapy.Citation2,Citation12 We believe that the etiology of amyloidosis is the third important factor for prediction of colchicine response.
In conclusion, colchicine is an effective medication in the prevention and treatment of amyloidosis due to FMF. The most beneficial effect of the therapy is observed in the early stages of the disease. However, the beneficial effect of this therapy is suspicious for other RD-associated amyloidosis. The initiation of immunosuppressant and biologically active drugs may improve amyloidosis and prevent mortality and morbidity in the early phase of RD-associated amyloidosis. We need further studies for defining effect and timing of alternative treatments in RD-associated amyloidosis.
Declaration of interest
We declare that there is no conflict of interest between authors, and no financial support.
References
- Ozturk MA, Kanbay M, Kasapoglu B, et al. Therapeutic approach to familial Mediterranean fever: a review update. Clin Exp Rheumatol. 2011;29:77–86
- Oner A, Erdoğan O, Demircin G, et al. Efficacy of colchicine therapy in amyloid nephropathy of familial Mediterranean fever. Pediatr Nephrol. 2003;18:521–526
- Sevoyan MK, Sarkisian TF, Beglaryan AA, et al. Prevention of amyloidosis in familial Mediterranean fever with colchicine: a case-control study in Armenia. Med Princ Pract. 2009;18:441–446
- Zemer D, Pras M, Sohar E, et al. Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever. N Engl J Med. 1986;314:1001–1005
- Cakar N, Yalçinkaya F, Ozkaya N, et al. Familial Mediterranean fever associated amyloidosis in childhood. Clinical features, course and outcome. Clin Exp Rheumatol. 2001;19:63–67
- Kaaroud H, Boubaker K, Béji S, et al. Renal amyloidosis followed more than 5 years: report of 12 cases. Transplant Proc. 2004;36:1796–1798
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470
- Saatçi U, Ozen S, Ozdemir S, et al. Familial Mediterranean fever in children: report of a large series and discussion of the risk and prognostic factors of amyloidosis. Eur J Pediatr. 1997;156:619–623
- Ravid M, Robson M, Kedar I. Prolonged colchicine treatment in four patients with amyloidosis. Ann Intern Med. 1977;87:568–570
- Simşek B, Işlek I, Simşek T, Küçüködük S, Cengiz K. Regression of nephrotic syndrome due to amyloidosis secondary to familial Mediterranean fever following colchicine treatment. Nephrol Dial Transplant. 2000;15:281–282
- Rozenbaum M, Rosner I. Regression of amyloidosis with colchicine in familial Mediterranean fever in an Ashkenazi patient. Clin Exp Rheumatol. 1995;13:126
- Livneh A, Zemer D, Langevitz P, Laor A, Sohar E, Pras M. Colchicine treatment of AA amyloidosis of familial Mediterranean fever. An analysis of factors affecting outcome. Arthritis Rheum. 1994;37:1804–1811
- Sarkissian A, Papazian M, Sanamyan A, Leumann E. Colchicine in the treatment of renal amyloidosis secondary to familial Mediterranean fever. Nephrol Dial Transplant. 2000;15:1098
- Bergesio F, Ciciani MA, Santostefano M, et al. Renal involvement in systemic amyloidosis – an Italian retrospective study on epidemiological and clinical data at diagnosis. Nephrol Dial Transplant. 2007;22:1608–1618
- Immonen K, Finne P, Hakala M, Kautiainen H, Pettersson T, Grönhagen-Riska C. No improvement in survival of patients with amyloidosis associated with inflammatory rheumatic diseases data from the Finnish national registry for kidney diseases. J Rheumatol. 2008;35:1334–1338
- Teilum G. Pathogenesis of amyloidosis. Acta Pathol Microbiol Scand. 1964;61:21–45
- Sipe JD, McAdam KP, Uchino F. Biochemical evidence for the biphasic development of experimental amyloidosis. Lab Invest. 1978;38:110–114
- Shirahama T, Cohen AS. Redistribution of amyloid deposits. Am J Pathol. 1980;99:539–550
- Kuczynski M. Further contributions to the principle of amyloid. Klin Wochenschr. 1923;2:2193–2195
- Hawkins PN, Pepys MB. A primed state exists in vivo following histological regression of amyloidosis. Clin Exp Immunol. 1990;81:325–328
- Obici L, Merlini G. AA amyloidosis: basic knowledge, unmet needs and future treatments. Swiss Med Wkly. 2012;142:13580
- Nowak B, Jeka S, Wiland P, Szechiński J. Rapid and complete resolution of ascites and hydrothorax due to nephrotic syndrome caused by renal amyloidosis in a patient with juvenile chronic arthritis treated with adalimumab. Joint Bone Spine. 2009;76:217–219
- Smith GR, Tymms KE, Falk M. Etanercept treatment of renal amyloidosis complicating rheumatoid arthritis. Internal Med J. 2004;34:570–572
- Tweezer-Zaks N, Langevitz P, Livneh A. Long-term followup needed to define role of infliximab in treatment of renal amyloidosis: comment on the case report by Elkayam et al. Arthritis Rheum. 2003;48:3298–3299