Abstract
Calcification of coronary vessels progresses rapidly in hemodialysis (HD) patients and comprises a strong predictor of cardiovascular events. The aim of this prospective study was to evaluate the coronary artery calcification (CAC) in patients with end stage renal disease undergoing regular HD and to determine the effect of renal transplantation (RT) in the progression of CAC, using the Agatston technique for calcium scoring. The study included 20 patients with end-stage renal disease undergoing a regular HD treatment (16 males, 4 females) 54.1 ± 9.5 years old who had just received a renal transplant and 16 more HD patients (11 males, 5 females) 54.4 ± 13.8 years old as control group. The baseline evaluation showed a very high prevalence of CAC in both groups, which was positively correlated with age (p < 0.001) and CRP (p = 0.03). The second (follow-up) evaluation showed a significant slower progression of calcification after RT. In both groups, high calcium score values in the follow-up evaluation had a strong positive correlation with baseline calcium score (p < 0.001).
Introduction
Disturbances in mineral and bone metabolism are prevalent in patients with chronic kidney disease (CKD) and are an important cause of morbidity, decreased quality of life and extra-skeletal calcifications.Citation1
Calcification of coronary vessels comprises a major component of extra-skeletal calcification. It develops early and progresses rapidly in hemodialysis (HD) patients.Citation2 Moreover, several studies have showed that vascular calcification, comprises a strong predictor of cardiovascular events,Citation3,Citation4 which are the leading cause of premature death in these patients.Citation5
Although several reports support that renal transplantation (RT) and restoration of renal function slow down the calcification process,Citation6,Citation7 the data regarding the evolution of calcification of coronary vessels after RT remain conflicting.
The aim of this prospective study was to evaluate the coronary artery calcification (CAC) in patients with end-stage renal disease (ESRD) undergoing regular HD, to identify the factors that are associated with high CAC and finally to determine the effect of RT in the progression of CAC.
Patients and methods
The study included 20 patients with ESRD undergoing a regular HD treatment (16 males, 4 females) 54.1 ± 9.5 years old who had just received an RT, and 16 more HD patients (11 males, 5 females) 54.4 ± 13.8 years old as control group. Other laboratory values and clinical characteristics of both groups are shown in . Regarding the PTH values, in HD group only 1 patient had PTH < 100 pg/mL and 2 patients had 100 < PTH < 150 pg/mL. In RT group, only 1 patient had PTH values less than 100 pg/mL and 3 patients had 100 < PTH < 150 pg/mL. Although this is suggestive of a low prevalence of low turnover bone disease, this was not established by bone biopsy and a mixed bone disease cannot be excluded. The protocol was approved by the Ethics Committee of the Hospital and a written informed consent was obtained from all patients. At the time of the study all patients were on HD 4 h thrice a week for 56 ± 34 months. All RT patients were given an induction therapy with anti-CD25 antibodies (basiliximab), and during follow-up period they were under a triple immunosuppression regimen including prednisolone, a calcineurin inhibitor (cyclosporine A or tacrolimus) and mycophenolate mofetil (ΜΜF). Despite the fact that calcineurin inhibitors may cause abnormalities of the lipid metabolism, cholesterol levels did not show any significant increase in RT group during the follow-up period. Initially, for the evaluation of CAC, all patients underwent a baseline multislice spiral coronary computed tomography (MSCT) using the Agatston technique for calcium scoring (CS).Citation8 Left main, left anterior, circumflex, right coronary artery, posterior descending artery, as well as main branches of them (diagonal, obtuse marginal) were evaluated for the presence of calcified plaques and the calcium score (CS) was categorized as follows: (a) CS = 0 corresponding to no identifiable plaque and a very low (generally <5%) risk of coronary artery disease, (b) 1 ≤ CS ≤ 10 corresponding to a minimal identifiable plaque and a risk of coronary artery disease less than 10%, (c) 11 ≤ CS ≤ 100 corresponding to mild atherosclerotic plaque and existence of mild or minimal coronary narrowing, (d) 101 ≤ CS ≤ 400 corresponding to at least moderate atherosclerotic plaque and possible significant narrowing of coronary arteries and (e) CS ≥ 401 corresponding to extensive atherosclerotic plaque and a high likelihood of at least one significant coronary narrowing.
Table 1. Patients’ demographic characteristics and most important laboratory parameters.
The examination was performed using a commercially available 16-slice CT scanner (Aquilion 16, Toshiba) with a gantry rotation time of 420 msec. Patients who had heart rates >70 received a beta-blocker (50 mg metoprolol once). A routinely used scanning protocol for coronary artery calcium quantification was used (section thickness, 3 mm; collimation, 1.5 mm; table feed, 5.7 mm per rotation; reconstruction increment, 1.5 mm).
Patients were positioned at the CT table in decubitus position and they were all electrocardiographically monitored. The entire heart was covered in a single breath. Prospective ECG-triggering was used and the images were acquired at a diastolic moment defined by the patient’s heart rate. To minimize interobserver variability of results, all computed tomographic scans were evaluated by the same investigator. Coronary calcifications were defined as the presence of more than two contiguous pixels with density greater than 130 HU. Baseline MSCT in the RT group was performed immediately after transplantation.
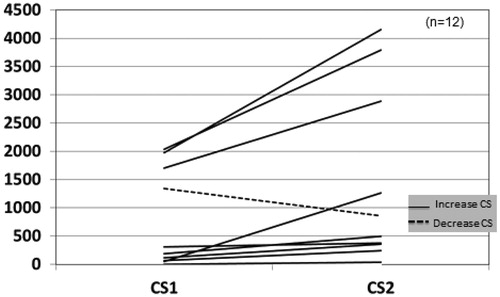
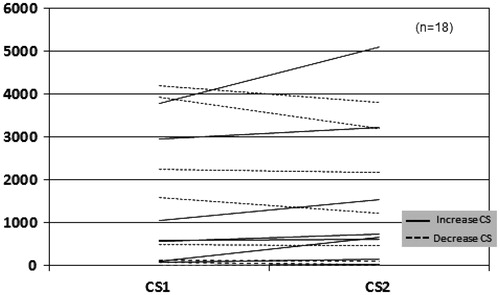
Serum parathyroid hormone (PTH), Ca, P, Mg, and Ca x P product, LDL and HDL cholesterol, and C-reactive protein (CRP) were measured in all patients once upon inclusion in the study. After a mean follow-up of 16 ± 4 months, 18 RT patients and 12 HD patients underwent a second MSCT in order to evaluate the progression of CAC.
Statistical analysis
Comparisons across baseline CAC of RT and HD group were performed with the Mann–Whitney U test, as this method was also applied for the rate of CS progression. The relationship between demographic data, laboratory and CAC among RT and HD sample population, was determined by Spearman rank analysis. Differences in baseline CS (CS1) and follow up CS (CS 2) between the RT and HD group, were separately analyzed by Wilcoxon test. A p value of ≤0,05 was considered to be statistically significant. All statistical analyses were performed using SPSS 17.0.1.
Results
In the baseline evaluation, only 5 (14%) patients were free of CAC (CS = 0). Six patients had 11 ≤ CS ≤ 100, 7 patients had severe CAC with 101 ≤ CS ≤ 400 and 18 (50%) patients suffered from very severe CAC with CS > 400, corresponding to a high likelihood of at least one significant coronary narrowing. The baseline CS in both groups, was positively correlated with age (p < 0.001) and CRP (p = 0.03) but no significant association was found between baseline CS and the measured laboratory values. Two RT patients and one HD patient did not complete the study because of personal reasons, and three more HD patients deceased before the end of the study, (two from coronary heart disease and one from severe infection). In HD patients, follow-up CS (CS2) was significantly increased (p = 0.028; ), whereas RT group did not show significant change (). The rate of CS progression was significantly slower in RT group than in HD group (p = 0.05). Seven RT recipients and one HD patient manifested an important decrease in CS. In RT group, although most patients manifested a delayed graft function (DGF) and needed to undergo some HD sessions, finally all patients have retrieved a glomerular filtration rate (GFR) between 30 and 59 mL/min (stage 3a–3b of CKD). However, no significant association was found between the low CS2 and creatinine values (1.43 ± 0.7 mg/dL, range 0.74–2.8 mg/dL). In both groups, high CS2 values had a strong positive correlation with high baseline CS (p < 0.001) and most of the patients without any CAC in the first evaluation continued to remain free of calcification burden (CS2 = 0). Phosphate, calcium and PTH during the follow up period were evaluated in both groups, but they did not show any significant change, except for phosphate in RT group (p = 0.001).
Discussion
CKD-Mineral and Bone Disorders entity (CKD-MBD) describes a broad clinical syndrome that develops as a systemic disorder of mineral and bone metabolism due to CKD and is manifested by abnormalities in bone and mineral metabolism and/or extra-skeletal calcification.Citation1 Extra-skeletal calcifications and particularly calcification of coronary vessels develop early and progress rapidly in HD patients, even in young adults.Citation2 Elevated serum phosphate levels seem to play an important role in the vascular calcification process, even in pre-dialysis CKD patients and despite being within the normal range.Citation9 The hyperparathyroidism and the chronic inflammation status (expressed by elevated serum CRP levels) have been strongly associated with the presence of CAC.Citation7
In the general population, CAC is normally only seen in advanced atherosclerotic plaques and is very rarely seen in normal vessels. Measurement of CAC by electron-beam computerized tomography (EBCT) correlates with total plaque burden and is predictive of future ischemic events and outcome.Citation10 However, there are several reports supporting that CAC measured by EBCT is not an accurate marker of the degree of vessel stenosis in coronary artery disease in uremic patients.Citation11,Citation12 Indeed, vascular calcification may involve either the intima, in association with inflammation and atherosclerosis, or the media, causing vascular stiffness. Both processes often coexist in HD patients and cannot be distinguished by imaging techniques, including computed tomography.Citation13 However, vascular calcification in general is responsible for many HD complications and remains a strong predictor of cardiovascular and all-cause mortality in ESRD patientsCitation3 and also in renal transplant recipients.Citation14
Regarding the evolution of vascular calcification after RT, the data remain conflicting. There are several reports supporting the regression of coronary calcification process after RT, due to improvement in mineral metabolism and elimination of uremic toxins.Citation6,Citation7 In fact, Oschatz et al. found that the slowdown of calcification process occurs mainly after the sixth month after RT.Citation15 However, a recent large study of 281 patients failed to demonstrate any benefit regarding coronary calcification after RT.Citation16
In the present study, baseline CS in both groups correlated significantly with age (p < 0.001) and CRP (p = 0.03). Although it was not associated with any of the measured values of mineral metabolism parameters this was probably due to the small number of patients. Moreover, the vascular calcification process involves a mechanism which is more complex than a passive calcium precipitation on vascular walls. Bargnoux et al. found that baseline osteoprotegerin (OPG) levels could have a predictive value for calcification progress, but with no significant association.Citation6 Others natural protective substances such a pyrophosphate, fetuin-A levels, bone morphogenetic protein-7 (BNP7) and matrix gla proteins could play an inhibitory role but unfortunately were not evaluated in the present study. Some medications such as warfarin, also promote the calcification process by deranging matrix gla protein metabolism. However, none patient was on such a regimen. Although baseline CS and other initial laboratory values were not significantly different between the RT group and the HD group, CS tends to increase significantly in the HD group (p = 0.028; ), whereas it did not show significant change in RT group. Hence, there was a positive correlation of RT with a slower rate of CAC progress and a lower CS2 (p = 0.05). Despite the fact that there was no statistically significant change in CS in RT group, it is remarkable that seven patients manifested an important decrease of CS. Although it seems rational to expect a lower CS in patients with better renal function, no important association was found between them. Surprisingly, the patients with lower CS had not the best renal function, but probably this fact indicates the complex nature of the vascular calcification process. However, and despite the fact that the initial evaluation did not highlight any association of CS with mineral metabolism laboratory parameters, a marked improvement of phosphate levels (pre-transplantation phosphate: 5.4 ± 1.2 mg/dL, post-transplantation phosphate: 2.4 ± 0.4 mg/dL; p: 0.001) could contribute to calcification reduction, given that high phosphate levels are said to be associated with induction of some osteoblast specific genes and promote vascular smooth cell calcification.Citation17 In contrast, only one patient from HD group, who had been on a bisphosphonates regimen because of glucocorticoid induced osteoporosis, manifested a decline in CS (CS2< baseline CS). This finding is in accordance with some evidence supporting the decrease of extra-skeletal calcification by bisphosphonates.Citation18 In HD patients, although calcium containing phosphate binders, have been implicated for vascular calcification, only a minority (two patients) were on such a regimen. All HD patients were on therapy with alphacalcidol and two HD patients were taking cinacalcet, but no association was found between CS and medical treatment, but the numbers were small to reach certain significance.
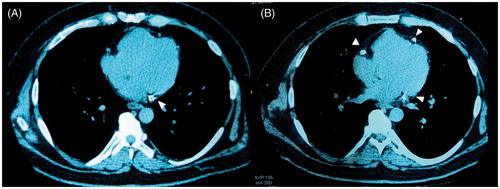
Figure 3. (A) Baseline MSCT and (B) Follow-up MSCT of a HD patient. There is a significant increase of coronary artery calcifications (arrowheads). MSCT: multislice computed tomography, HD: hemodialysis, CS: calcium score.
Regarding mortality, although it was higher in HD group than in RT group (three deaths versus none), we consider that such a conclusion would be unreliable because of the small number of patients. The most important finding of the study was the finding that the follow up CS was significantly correlated to the baseline CS.
Conclusions
The present study prospectively shows that the prevalence of CAC in HD patients is very high and progresses during time and is mostly associated with age and CRP level. RT and recovery of renal function considerably halted CAC progression. Very important was the finding that the follow-up CS was significantly related to the baseline CS, which emphasizes the importance of primary prevention of CAC development.
Limitations of the study
The major limitation of the study is the small number of patients and the fact that all parameters were obtained once upon inclusion in the study.
References
- Moe S, Drüeke T, Cunningham J, et al. Kidney Disease: improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945–1953
- Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483
- Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942
- Vliegenthart R, Hollander M, Breteler MM, et al. Stroke is associated with coronary calcification as detected by electron-beam CT: the Rotterdam Coronary Calcification Study. Stroke. 2002;33:462–465
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119
- Bargnoux AS, Dupuy AM, Garrigue V, et al. Evolution of coronary artery calcifications following kidney transplantation: relationship with osteoprotegerin levels. Am J Transplant. 2009;9:2571–2579
- Mazzaferro S, Pasquali M, Taggi F, et al. Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation. Clin J Am Soc Nephrol. 2009;4:685–690
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte Jr M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832
- Stavroulopoulos A, Porter CJ, Pointon K, Monaghan JM, Roe SD, Cassidy MJ. Evolution of coronary artery calcification in patients with chronic kidney disease Stages 3 and 4, with and without diabetes. Nephrol Dial Transplant. 2011;26:2582–2589
- Keelan PC, Bielak LF, Ashai K, et al. Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation. 2001;104:412–427
- Sharples EJ, Pereira D, Summers S, et al. Coronary artery calcification measured with electron-beam computerized tomography correlates poorly with coronary artery angiography in dialysis patients. Am J Kidney Dis. 2004;43:313–319
- Tong LL, Mehrotra R, Shavelle DM, Budoff M, Adler S. Poor correlation between coronary artery calcification and obstructive coronary artery disease in an end-stage renal disease patient. Hemodial Int. 2008;12:16–22
- Amann K. Media calcification and intima calcification are distinct entities in CKD. Clin J Am Soc Nephrol. 2008;3:1599–1605
- Nguyen P, Henrard S, Coche E, Goffin E, Devuyst O, Jadoul M. Coronary artery calcification: a strong predictor of cardiovascular events in renal transplant recipients. Nephrol Dial Transplant. 2010;25:3773–3778
- Oschatz E, Benesch T, Kodras K, Hoffmann U, Haas M. Changes of coronary calcification after kidney transplantation. Am J Kidney Dis. 2006;48:307–313
- Maréchal C, Coche E, Goffin E, et al. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am J Kidney Dis. 2012;59:258–269
- Giachelli CM, Jono S, Shioi A, Nishizawa Y, Mori K, Morii H. Vascular calcification and inorganic phosphate. Am J Kidney Dis. 2001;38:S34–S37
- Hashiba H, Aizawa S, Tamura K, Shigematsu T, Kogo H. Inhibitory effects of etidronate on the progression of vascular calcification in hemodialysis patients. Ther Apher Dial. 2004;8:241–247