Abstract
Objective: This study evaluated the usefulness of plasma Cystatin C (pCysC) along with urinary neutrophil gelatinase-associated lipocalin (NGAL), γ-glutamyltransferase (GGT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), aspartate (AST) and alanine (ALT) aminotransferase to monitor colistin nephrotoxicity. Method: Male rats were given intramuscular (i.m.) injections of colistin in doses of 150,000 (G1), 300,000 (G2) and 450,000 IU/kg/day (G3) or normal saline (Control), every 12 h for 7 days. After the 14th injection, animals were placed in metabolic cages and urine samples were collected in the next 12 h. Thereafter, animals were euthanized, blood samples were collected and kidneys were removed for histological assessment. Results: Nephrotoxicity was completely dose-dependent according to pathologic findings. The major insults were acute tubular necrosis in the tubules of G3. No significant change in pCr was observed in all treated groups, but pCysC increased in the G3 compared to the control. In urinary markers, uNGAL level showed a dose dependant increase with significant change in the G2 and G3 groups compared to the control. However, there was no significant change in the AST, ALT, LDH or ALP activities but only GGT increased in the G3 compared to the control.
Conclusion: Based on colistin doses used in our experimental study on rat model, histopathologic assessment remains the most accurate way to diagnose colistin nephrotoxicity. pCysC appears to be more reliable than pCr, and uNGAL seems to be the most sensitive factor of colistin nephrotoxicity.
Introduction
Colistin, a polymyxin E, has a significant postantibiotic effect on multidrug-resistant gram-negative bacteria (MDR GNB), such as Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae. Since its first introduction in 1952, colistin has been mainly used to treat GNB. However, due to its renal toxicity, colistin has been abandoned in the last two decades.Citation1 Nevertheless, the increasing prevalence of infections by MDR GNB and the shortage of novel antibiotics, colistin was re-introduced into clinical practice as the last therapeutic option. The main mechanism of colistin-induced nephrotoxicity is acute tubular necrosis, causing a reduction in glomerular filtration and a renal failure,Citation2 which is manifested by an increase in plasma creatinine (pCr) level.Citation1 Cr is the conventional biomarkers used to evaluate kidney function. In addition, several urinary enzymes, such as alkaline phosphatase (ALP),Citation3 γ-glutamyltransferase (GGT),Citation4 lactate dehydrogenase (LDH),Citation5 aspartate (AST) and alanine aminotransferase (ALT)Citation6,Citation7 have been used as nephrotoxic biomarkers. Moreover, some low molecular weight proteins such as Cystatin C (CysC) and neutrophil gelatinase associated lipocalin (NGAL) emerged in animal and human studies as biomarkers of acute kidney injury (AKI). CyC is a monoglycosylated protein that is derived from the cystatin superfamily of cysteine protease inhibitors.Citation8 It is produced by all nucleated cells and is almost completely reabsorbed and catabolized by proximal tubular cells.Citation9 Serum CyC seems to be more sensitive than pCr to evaluate kidney damage and considered as alternative marker for use in renal function monitoring and drug dosage adjustment.Citation8,Citation9 NGAL has also been identified as a promising marker of renal failure,Citation10 and recently has been proposed as a marker of tubular dysfunction.Citation11 NGAL was markedly induced in kidney tubule cells and easily detected in the urine and plasma in animal models of ischemic and nephrotoxic AKI.Citation10,Citation11 In the case of colistin induced nephrotoxicity and to the best of our knowledge, little research has been used to detect renal damage by enzymuria. This study was therefore aimed to evaluate the usefulness of pCyC along with urinary NGAL, AST, ALT, LDH, ALP and GGT to monitor colistin nephrotoxicity, other than pCr. We used rats models of kidney injury induced by colistin; the maximal human therapeutic dose = 150,000 IU/kg/day and two high doses; 300,000 IU/kg/day and 450,000 IU/kg/day administered intramuscularly in twice daily doses (12 h apart) for 7days.
Material and methods
Drug
Clinically, colistin is administered parenterally as sodium colistin methanesulphonate (CMS), an inactive pro-drug that is converted into colistin, the antibacterial and toxic entity. CMS was from Aventis, France (1 million IU/vial).
Animals
Male Wistar rats weighing 200 ± 10 g were purchased from the breeding center of the Central Pharmacy (SIPHAT). All animal procedures were conducted in strict conformity with the local Institute Ethical Committee Guidelines for the Care and Use of laboratory animals of our institution: they were kept in an environmentally controlled breeding room (temperature: 22 ± 2 °C, humidity: 60 ± 5%, 12 h dark/Light cycle). All rats had free access to tap water and food. The rats were weighed prior to the first injection, every other day in order to adjust the dose according to weight changes and on the 7th day to measure weight changes during the study.
Experimental design
Animals were randomly divided into four groups (n = 6 each) as follows:
The control group (control) was injected with 1 mL/kg of intramuscular (i.m.) sodium chloride 0.9%.
Group1 (G1) received i.m. colistin [colistimethate sodium (CMS); 1 million IU/vial, Aventis, France] in doses of 150,000 IU/kg/day = 12 mg/kg/day, administered in twice daily doses (12 h apart) for 7days. The dose is equivalent to maximal therapeutic dose in healthy humans.Citation12
Group 2 (G2) was received i.m. colistin in doses of 300,000 IU/kg/dayCitation13,Citation14 = 24 mg/kg/day (a high dose, no corresponding human regimen) administered in twice daily doses (12 h apart) for 7days.
Group 3 (G3) was received i.m. colistin in doses of 450,000 IU/kg/day = 36 mg/kg/day administered in twice daily doses (12 h apart) for 7days. The doses selection was related to the higher protein bounding in rat (55%)Citation15 than in human (15%),Citation1 so larger doses of colistin in rat model are necessary to have the same pharmacodynamic effects and to increase, on the other hand, the chance of creating a renal toxicity.
Interestingly enough, 1 mg CMS is equivalent to 0.41 mg of colistin and that 1 mg CMS is equivalent to 12,500 IU CMS.
Urine sample preparation
After the 14th injections of colistin or normal saline, animals were housed in metabolic cages and 12 h urine were collected, centrifuged immediately after collection (1000 g, 5 min, +4 °C) to discard debris. The supernatant was aliquoted into two Eppendorf tubes, one tube was used for immediate determination of Cr, AST, ALT, LDH, ALP and GGT and the second was frozen at −80 °C for determination of NGAL level.
Blood sample preparation
After the last injection of colistin or normal saline, animals were anesthetized by an intraperitoneal injection of ketamine, and euthanized. The trunk blood was collected and 2 mL of blood were distributed into heparin tubes, and centrifuged at 4000 rpm for 15 min. The plasma was aliquoted into two Eppendorf tubes, one tube was used for immediate determination of pCr and the second was frozen at −80 °C for determination of pCysC.
Urinalysis and blood chemistry measurements
The concentration of Cr in both plasma and urine samples was measured by Jaffe method using commercial diagnostic kits (Ref. 304331) purchased from Biomagreb (Ariana, Tunisia).
Estimation of urinary AST, ALT, LDH, ALP and GGT activities were determined by enzymatic methods using commercial reagent kits (Refs. 304410, 304446, 304441, 304338 and 94-1328, respectively) from Biomaghreb (Ariana, Tunisia).
Urinary NGAL and plasma CysC levels were measured using a commercially available quantitative sandwich ELISA kit (BioVendor-Laboratorni medecina a.s.).
Histopathological examination
For light microscopic examination, kidney samples were fixed in 10% neutral buffered formalin, dehydrated in an ascending ethanol series (70%, 90% and 100%), cleared in xylene and embedded in paraffin. Paraffin sections (5 μm) were stained with hematoxylin and eosin using a routine method. The samples were examined by a pathologist (P. A. Hela Mniff) who was blinded to the treatment groups. Lesions were rated as follows:Citation16 grade 1, mild acute tubular damage with tubular dilation, prominent nuclei, and a few pale tubular casts; grade 2, severe acute tubular damage with necrosis of tubular epithelial cells and numerous tubular casts; and grade 3, acute cortical necrosis/infarction of tubules and glomeruli with or without papillary necrosis. The grades were given the following scores: grade 1 = 1, grade 2 = 4, and grade 3 = 10. The percentages of the kidney slices affected were scored as follows: <1% = 0; 1% to <5% = 1; 5% to <10% = 2, 10% to <20% = 3, 20% to <30% = 4; 30% to <40% = 5 and <40% = 6. The overall score was calculated as the product of percentage score and grade score. Finally, a semi-quantitative score (SQS) for renal histological changes was assigned as follows: SQS 0 = no significant change (overall score, <1); SQS + 1 = mild damage (overall score, 1 to <15); SQS + 2 = mild to moderate damage (overall score, 15 to <30); SQS + 3 = moderate damage (overall score, 30 to <45); SQS + 4 = moderate to severe damage (overall score, 45 to <60); and SQS + 5 = severe damage (overall score, 60).
Statistical analysis
Data are expressed as mean ± SD (standard deviation). The statistical significance between experimental groups was assessed by one-way analysis of variance (ANOVA) followed by Tukey post-hoc test. Results were considered statistically significant when p < 0.05.
Results
Effect of colistin dosage on blood markers
As reported in , there was no significant change in pCr levels in rats treated with colistin in doses of 150,000 IU/kg/day, 300,000 IU/kg/day or 450,000 UI/kg/day. There were no significant changes in pCysC in the 150,000 IU/kg/day and 300,000 IU/kg/day colistin groups compared to the control. Conversely, the 450,000 UI/kg/day group showed a significant increase (p < 0.01) in pCysC compared to the control.
Table 1. Plasmatic level of Cr and CysC in the control and treated rats with colistin in doses of 150,000, 300,000 or 450,000 (IU/kg/day) over 7days.
Effect of colistin dosage on urinary markers
shows no significant change in urinary AST, ALT, LDH or ALP activity in all colistin treated groups compared to the control. GGT activity increased (p < 0.05) in the 450,000 IU/kg/day group. As for uNGAL, it revealed a dose dependent increase, with significant change in the 300,000 (p < 0.05) and 450,000 IU/kg/day (p < 0.01) groups, compared to the control.
Table 2. Urinary level of AST, ALT, LDH, ALP, GGT and NGAL in the control and treated rat with colistin in doses of 150,000, 300,000 or 450,000 (IU/kg/day) over 7days.
Effect of colistin dosage on kidney histopathogy
Kidneys of rats treated with saline for 7 days showed normal renal cortex and glomeruli ( and ). Histological analysis of kidney samples of rats treated with colistin in doses of 150,000 IU/kg/day showed only minor renal damage with a few local tubular vacuolizations ( and ). In rats treated with colistin in doses of 300,000 IU/kg/day, we noted slight tubular dilatation and epithelial cell vacuolations ( and ). However, in the third experimental group treated with colistin in doses of 450,000 IU/kg/day we found kidney tubular lesions in all animals, without apparent glomerular lesions ( and ). These tubular injuries are described in vacuolar degeneration and tubular epithelial cells necrosis with numerous casts, but without inflammatory reaction or fibrous cicatrization ( and ).
Figure 1. Kidney of control rat treated with saline for 7 days showing normal renal cortex and glomeruli (HE × 100).
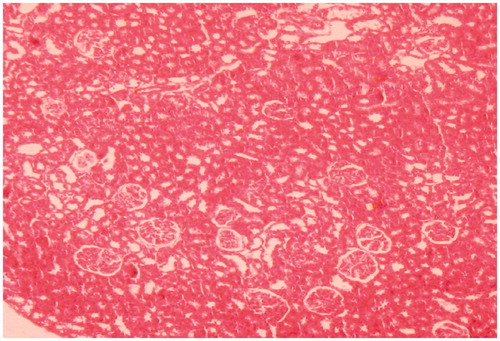
Figure 2. Kidney of rat administered colistin in doses of 150,000 IU/kg/day for 7 days showing mild tubular damage with vacuolisation (HE × 100).
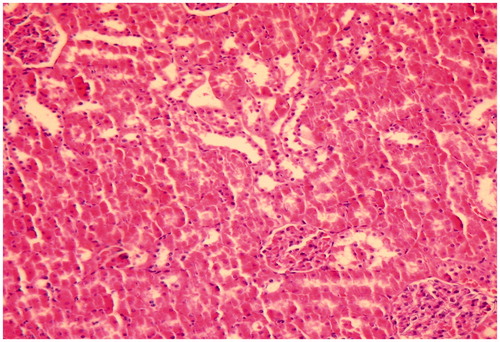
Figure 3. Kidney of rat administered colistin in doses of 300,000 IU/kg/day showing mild tubular dilatation with few pales casts (HE × 200).
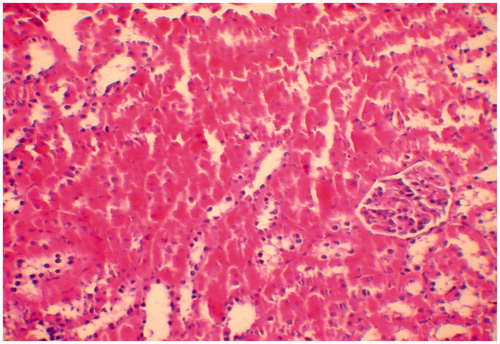
Figure 4. (A) Kidney of rat administered colistin in doses of 450,000 IU/kg/day showing acute tubular necrosis with glomerular preservation (*) (HE × 200). (B) Kidney of rat administered colistin in doses of 450,000 IU/kg/day showing acute cortical necrosis with numerous tubular casts (↗) (HE × 400).
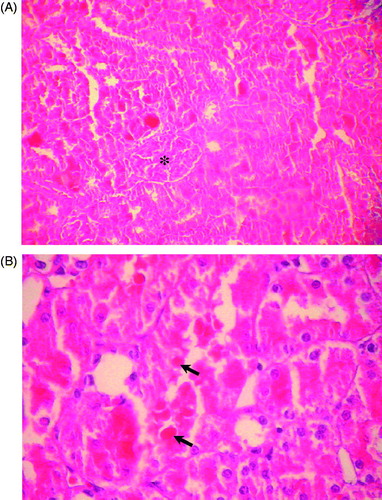
Table 3. Histological examination of renal lesions of colistin treated rats over 7 days.
Discussion
Nephrotoxicity represents a common serious clinical problem of colistin treatment which is conventionally determined by a rise in serum Cr concentration. However there are important limitations to this approach, and there has been interest in alternative biomarkers that might provide a more sensitive and rapid means of detecting acute kidney injury. In our present study, we used the histological kidney changes and the following biomarkers: sCysC, urinary NGAL and AST, ALT, LDH, ALP and GGT to monitor at different doses colistin nephrotoxicity in rat model.
Histopathologically, our present study demonstrated that colistin induced a proximal tubular injury in kidneys of rats treated with 300,000 and 450,000 IU/kg/day accompanied by a rise in urine NGAL and GGT levels but not in pCr or urinary AST, ALT, LDH and ALP. However, in 150,000 IU/kg/day group, there was no significant change either in kidney pathology (only two of the six rats showed a minor evidence of renal damage with a few local tubular vacuolization) or in biochemical parameters ( and ).
Kidneys of rats which received 300,000 IU/kg/day of CMS showed a mild acute tubular damage with tubular dilatation, prominent nuclei and a few pale tubular casts ( and ). Furthermore, in doses of 450,000 IU/kg/day, colistin induced a tubular epithelial cells necrosis with numerous casts. It therefore seems that histological damage is dose-dependent, which confirms previous statements regarding the dose dependency of colistin-induced nephrotoxicity.Citation17 Moreover, based on our data, we may further assume that histopathological changes in 300,000 IU/kg/day (24 mg/kg/day) and 450,000 IU/kg/day (36 mg/kg/day) were more reliable to detect colistin nephrotoxicity than pCr. Our results are in line with those of Wallace et al.Citation18 who showed no significant change in pCr level after 30 mg/kg/12 h of colistin over 7days.
In the 450,000 IU/kg/day group, pCysC concentration exhibited a significant rise, without pCr change (). CysC are known as functional biomarkers because they can freely pass through glomerular capillary walls due to their low-molecular weight and are almost completely reabsorbed by epithelial cells in proximal tubules.Citation19 The increased level of pCys could be therefore explained by filtral glomerular deterioration. The latter can be attributed either to a direct functional change in the glomeruli and/or secondary with acute tubular necrosis generated by colistin,Citation20 causing necrotic cell desquamation and an accumulation of insoluble protein substances within the tubular lumina. This will induce a decrease in urinary flow, a reduction in glomerular filtration and a renal failure.Citation2,Citation21 Histopathologically, our results are congruent with the second supposition, because we noted a markedly diffused tubular damage in the kidneys of rats treated with colistin in doses of 450,000 IU/kg/day, with tubular epithelial cells necrosis and numerous casts, but without apparent glomerular injury ( and ). pCysC could therefore prove to be a more reliable marker than pCr of colistin-induced nephrotoxicity.
The tubular damage observed in 300,000 IU/kg/day group did not cause disruption of glomerular function and did not change consequently pCysC, contrary to the 450,000 IU/kg/day group. Several urine parameters could be more sensitive for determination of renal damage. Among the markers evaluated in our study, uNGAL showed a dose-dependent elevation with histopathological evidence of tubular injury clearly observed in the second and third treated groups. In 300,000 IU/kg/day of colistin dose received by second group, we found a significant uNGAL increase, suggesting that this parameter is more sensitive than pCysC for the detection of renal damage induced by treatment with colistin. Several studies either experimental in ratsCitation22,Citation23 or clinical in patients with different pathologiesCitation24,Citation25 revealed the importance of early detection of kidney lesions induced by drug or other nephrotoxic factors. Urine NGAL has also been shown to predict the severity of AKI and over-expressed in response to injury.Citation26,Citation10 In our study, the uNGAL level rose according to renal lesion. In addition, an explanation for the uNGAL rise is that reabsorptive function of the proximal tubule declined due to progressive tubular damage. It has been reported that urine NGAL increase is caused by the inhibition of its tubular reabsorption.Citation27 Urinary NGAL can be therefore considered a biomarker of proximal tubular injury. However, future research is necessary to further define the predictive role of urine NGAL in colistin-induced nephrotoxicity.
As to the other urinary biomarkers assayed, GGT activity showed a significant increase in the third treated group, without any change in urinary AST, ALT, LDH or ALP activities (). GGT is located in the brush border of the proximal tubular epithelium. The urine increase in the concentration of this enzyme denotes lesion in the brush border membrane.Citation28 In this study, we observed a high increase in the GGT activity especially in the third group. These results are in agreement with previous findings on vancomycin and gentamicin which demonstrate that urine activity of GGT increased in the setting of acute tubular necrosis due to excess cellular loss, and elevated urinary enzyme activity has been used to indicate proximal tubular injury.Citation29
Regarding colistin doses used in our experimental study on rat model (150,000, 300,000 and 450,000 IU/kg/day), we concluded that histopathologic assessment remains the most accurate way to diagnose colistin nephrotoxicity. pCysC appears to be more reliable than pCr, and uNGAL seems to be the most sensitive factor of colistin nephrotoxicity. It could be important to validate the sensitivity and specificity of these biomarkers in clinical samples with therapeutic doses and to establish whether they might offer improved clinical outcomes in the clinical toxicology setting.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors are grateful to Professor Bou Yahia Moufida for assistance in writing this article.
References
- Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin Infect Dis. 2005;40:1333–1341
- Markowitz GS, Perazella MA. Drug-induced renal failure: a focus on tubulointerstitial disease. Clin Chim Acta. 2005;351:31–47
- Kocaoglu S, Karan A, Berkan T, et al. Acute acetaminophen nephrotoxicity and urinary gamma-glutamyl transferase activity in rats. Drug Metabol Drug Interact. 1997;14:47–54
- Naghibi B, Ghafghazi T, Hajhashemi V, et al. Vancomycin induced nephrotoxicity in rats: is enzyme elevation a consistent finding in tubular injury? J Nephrol. 2007;20:482–488
- Gumbleton M, Nicholls PJ. Dose-response and time-response biochemical and histological study of potassium dichromate-induced nephrotoxicity in the rat. Food Chem Toxicol. 1988;26:37–44
- da Silva Melo DA, Saciura VC, Poloni JA, et al. Evaluation of renal enzymuria and cellular excretion as a marker of acute nephrotoxicity due to an overdose of paracetamol in Wistar rats. Clin Chim Acta. 2006;373:88–91
- Stonard MD, Gore CW, Oliver GJ, et al. Urinary enzymes and protein patterns as indicators of injury to different regions of the kidney. Fundam Appl Toxicol. 1987;9:339–351
- Herget-Rosenthal S, Marggraf G, Hüsing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–1122
- Herget-Rosenthal S, van Wijk JA, Bröcker-Preuss M, et al. Increased urinary cystatin C reflects structural and functional renal tubular impairment independent of glomerular filtration rate. Clin Biochem. 2007;40:946–951
- Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543
- Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024
- Garnacho-Montero J, Ortiz-Leyba C, Jimenez-Jimenez FJ, et al. Treatment of multidrug-resistant Acinetobacter baumanii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis. 2003;36:1111–1118
- Hakim A, Kallel H, Sahnoun Z, et al. Lack of nephrotoxicity following 15-day therapy with high doses of colistin in rats. Med Sci Monit. 2008;14:74–77
- Ozyilmaz E, Ebinc FA, Derici U, et al. Could nephrotoxicity due to colistin be ameliorated with the use of N-acetylcysteine? Intensive Care Med. 2010;37:141–146
- Li J, Milne RW, Nation RL, et al. Use of high performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother. 2003;47:1766–1770
- Yousef JM, Chen G, Hill PA, et al. Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob Agents Chemother. 2011;55:4044–4049
- Hoeprich PD. The polymyxins. Med Clin North Am. 1970;54:1257–1265
- Wallace SJ, Li J, Nation RL, et al. Subacute toxicity of colistin methanesulfonate in rats: comparison of various intravenous dosage regimens. Antimicrob Agents Chemother. 2008;52:1159–1161
- Conti M, Moutereau S, Zater M, et al. Urinary cystatin C as a specific marker of tubular dysfunction. Clin Chem Lab Med. 2006;44:288–291
- European Medicines Agency (EMEA). Final report on the pilot joint EMEA/FDA VXDs experience on quantification of nephrotoxicity biomarkers, Available at: www.emea.europa.eu/pdfs/human/biomarkers/2008/25088508en.pdf September 2010, Accessed May 6, 2010
- Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008;245:182–193
- Tonomura Y, Tsuchiya N, Torii M, et al. Evaluation of the usefulness of urinary biomarkers for nephrotoxicity in rats. Toxicology. 2010;273:53–59
- Sasaki D, Yamada A, Umeno H, et al. Comparison of the course of biomarker changes and kidney injury in a rat model of drug-induced acute kidney injury. Biomarkers. 2011;16:553–566
- Nguyen MT, Devarajan P. Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol. 2008;23:2151–2157
- Devarajan P. Emerging urinary biomarkers in the diagnosis of acute kidney injury. Expert Opin Med Diagn. 2008;2:387–398
- Kuwabara T, Mori K, Mukoyama M, et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75:285–294
- Koyner JL, Vaidya VS, Bennett MR, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5:2154–2165
- D’Amico G, Bazzi C. Pathophysiology of proteinuria. Kidney Int. 2003;63:809–825
- Whiting PH, Brown PA. The relationship between enzymuria and kidney enzyme activities in experimental gentamicin nephrotoxicity. Ren Fail. 1996;18:899–909
