Abstract
Introduction: Chronic kidney disease (CKD) is an important health care problem with increasing incidence. Early diagnosis, recognition and interventions to avoid the disease progression have great value. Even some risk factors for disease progression have been described; there are still some dark spots. Transforming growth factors (TGFs), particularly bone morphogenetic protein-7 (BMP7) take place in renal fibrosis. Our study aimed to evaluate the association between serum BMP7 levels and the progression of CKD. Materials and methods: Our study has been conducted between January 2008 and December 2010. Decrease in GFR by 10%, doubling of serum creatinine and need for renal replacement therapy have been set as progression end-points. Totally 93 patients (48 female, 45 male) have been included. Baseline and end of follow-up BMP7 levels have been measured. Results: At the end of the follow-up, 46 of 93 patients have been considered as having progressive CKD. Higher levels of serum BMP7 levels have been found to be associated in progressive kidney disease. Discussion: Our results showed that BMP7 levels were higher in patients with progressive CKD, and also BMP7 to be associated with CKD progression. But this relationship was not statistically significant. In patients with progressive CKD, higher levels of proteinuria and blood pressure have been previously described. The effect of BMP7 on kidneys is not still clear, it is hypothesized that TGF-beta1 inhibition may alter renal fibrosis.
Introduction
Early diagnosis and treatment as well as implementation of measures against disease progression have been shown to slow down or even prevent the development of end stage renal failure and associated detrimental effects in patients with CKD.Citation1,Citation2
Several risk factors have been implicated in the development and progression of renal diseases.Citation3 The discovery of new markers would have numerous benefits; may help identify individuals at risk for progression of CKD, aid in the early detection of poor outcomes related to CKD (cardiovascular disease, need for RRT, decrease in quality of life and death), and help to better understand the mechanisms behind CKD progression.Citation4
The TGF family is widely believed to play a pivotal role in the development of renal fibrosis. The BMP7 molecule, a member of the TGF family, is essential for the embryological development of the kidney, and has been reported to play a role in the development of renal disorders.Citation5–7 In the only human study evaluating the relationship between serum BMP7 levels and renal function, significant reductions in BMP7 levels were observed in patients with contrast nephropathy.Citation8 We hypnotized that Serum BMP7 levels may be associated with the disease progression in patients with amyloidosis. In our study we aimed to investigate the association between particularly bone morphogenetic protein-7 (BMP7) and disease progression in renal amyloidosis patients.
Materials and methods
Patient selection and initial evaluation
This study was undertaken in the Department of Nephrology at Ankara Education and Research Hospital with the approval of the local ethics committee. Adult patient (age >18 years) with confirmed chronic renal disease, who were regularly followed up between January 2008 and December 2010, were approached for inclusion in the study and consenting patients were screened for eligibility. Any subject with suspected/confirmed pregnancy, elevated liver enzymes, signs of an active infection, a confirmed diagnosis of a malignant disorder, a disorder of uric acid metabolism and/or receiving vitamin D supplementation was excluded from the study. Also patients with uncontrolled hypertension, coronary arterial disease, anemia (Hb < 10 g/dL), thyroid dysfunction, diabetes mellitus or dyslipidemia were excluded. A detailed medical history was obtained from all participants followed by a careful physical examination. Findings on histopathological examination were recorded for any patients with a renal biopsy. Study design is shown in .
Blood sampling and laboratory assay
For the evaluation of baselines levels of study parameters, venous blood samples were obtained for all participants in the recumbent position from the antecubital region between 8.00 and 9.00 am after 8–12 hours overnight fast. Samples were sent for the immediate evaluation of complete blood count as well as for serum levels of alkaline phosphatase (ALP), calcium, phosphate, uric acid, albumin, C-reactive protein (CRP), and intact parathormone (PTH). Thyroid function tests (thyroid stimulating hormone, free thyroxine, and free triiodothyronine) and lipid profiles (total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and very low-density lipoprotein cholesterol) are measured. Glomerular filtration rates were calculated for each participant using the Modification of Diet in Renal Disease (MDRD) formula: [GFR (mL/min/1.73 m2) = 186 × (Pcr)−1.154 × (age)−0.203 × (0.742 if female)]. Participants were also required to collect 24-hour urine a day prior to blood sampling for the quantitative determination of proteinuria. All of the above-mentioned measurements at 6-months intervals for a total period of two years (total of 5 measurements, including baseline).
BMP7 measurements
The extracted serum samples from all participants were stored at −80 °C for the collective evaluation of BMP7 levels at the end of the study period. BMP7 level measurements were made using RayBio Human BMP7 Elisa kits. Unlike other laboratory parameters, BMP7 levels were measured twice – from samples obtained at baseline and by the end of the two-year follow-up period.
Definition of study outcomes
The primary endpoint of the study was progression of renal disease which was defined as a ≥10% decrease in estimated glomerular filtration rate (eGFR) or ≥0.5-fold increase in serum creatinine levels. These patients are divided into two groups and the ones who had progressive disease and did not have progressive disease were analyzed as group I and group II, respectively.
Statistical analysis
Statistical analyses were performed using SPSS 11.0 software. Values for categorical parameters were given as mean, whereas continuous numerical variables were provided as mean ± standard deviation. The extent of the relationship between findings on renal biopsy, baseline BMP7 levels, complete blood count parameters, serum alkaline phosphatase (ALP), thyroid function tests (TFT) and the effect of medication on renal disease progression was evaluated correlation analysis was performed between GFR and other parameters that were evaluated every 6 months (serum calcium, phosphorus, uric acid, albumin, lipid profile, CRP, PTH and response to medication). Differences between the groups analyzed using Student’s t-test, also Kruskal–Wallis and Mann–Whitney U-test when necessary. Categorical variables studied with chi-squared test.
Results
A total of 93 patients have been included in this study, all were biopsy-proven AA renal amyloidosis. Nine female and eleven male subjects have been evaluated with a mean age of 37 ± 12.2 years. Forty-six of 93 patients are considered having progressive loss of kidney functions more than 10% in eGFR values (group I). Baseline and end of follow-up serum creatinine and eGFR values and the difference ratios are listed in . Mean follow-up period of the patients was 24.4 ± 2.7 months.
Table 1. Baseline demographic characteristics and laboratory data of the entire study population (n = 93).
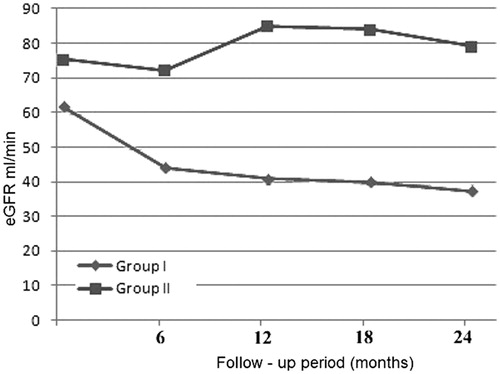
Baseline eGFR values of the patients who had progressive and non-progressive disease were not different with any statistical significance (62.5 ± 22.64 mL/min and 75.4 ± 26.03 mL/min respectively, p = 0.25). Serum BMP7 values of the participants were 436 ± 66.6 and 420 ± 92.2 in group I and group II, respectively, while these values were also similar (p = 0.48).
Baseline glomerular filtration rates were calculated as 62.5 ± 22.6 mL/min in group I and 75.4 ± 26.03 mL/min in group II (p = 0.25). At the end of the two-year follow-up period eGFR values were calculated as 38 ± 4.5 mL/min and 79.1 ± 24.1 mL/min in group I and group II, respectively (p < 0.05). eGFR changes of the groups is shown in .
When groups were compared in terms of serum BMP7 levels, at the end of the follow-up there was statistical significance. In group I BMP7 levels were higher than group II with statistical significance (522 ± 34.7 vs. 464 ± 89.4 p = 0.02). Baseline and follow-up values of the groups in terms of serum BMP7, creatinine and eGFR values are listed in .
Table 2. Baseline and follow-up values of the groups in terms of serum BMP7, creatinine and eGFR.
Discussion
Our results show that progression of CKD due to renal amyloidosis is relevant with the increase in serum BMP7 levels. Although it is widely believed that BMP7 is closely linked with the development of CKD, a review of the literature revealed very few studies investigating this relationship. In our study, disease progression was observed in 46 of 93 participants by the end of the two-years of follow-up period. In the MDRD study, several independent risk factors for the progression of CKD were established,Citation9 the most important of which being high proteinuria and hypertension.
The exact effect of BMP7 on adult kidneys remains unknown, although it is widely believed to be a potent inhibitor of TGFB1 and associated renal fibrosis.Citation7 Our study results show that BMP7 may be a potential marker in order to predict disease progression in patients with AA amyloidosis. However, the sample size in this study is quite limited, and there is a need for further research to help unequivocally establish a link between BMP7 renal AA amyloidosis, and perhaps shed some light on the pathogenic mechanisms behind this renal disorder.
Besides the known effects of elevated lipid levels in accelerating the systemic atherosclerotic process, hyperlipidemia has also been shown to result in progression of renal injury. Our data do not point such a relationship. Recently, other risk factors such as ADMA, FGF23, KIM-1, NGAL have also been described.Citation3
Previous studies have demonstrated a positive correlation between serum phosphate retention and disease progression.Citation10,Citation11 In two animal studies, restriction of dietary phosphate and the use of phosphate binders in rats had a protective effect on renal functions.Citation12,Citation13 Current data does not support the presence of a correlation between serum calcium levels and renal disease progression.Citation3 In a cohort study on 4231 patients with stage 4 CKD, elevated PTH levels were found to be associated with disease progression, along with male gender, younger age, lower hemoglobin levels, elevated systolic and diastolic blood pressure and high proteinuria.Citation7 Although we could not ascertain a link between baseline calcium and phosphate levels and diseases progression, baseline PTH levels were significantly higher in patients with progressive renal disease. In patients with progressive disease had lower serum calcium levels (p > 0.05) and higher serum phosphorus levels (p < 0.05).
Uric acid is assumed to be a novel risk factor for CKD and its progression. It has been demonstrated that hyperuricemia was significantly associated with the risk of developing CKD.Citation14,Citation15 Current data does not support the use of uric acid-lowering medications to prevent the progression of renal disease. In our study, patients with progressive renal disease had higher serum uric acid levels compared to patients with stable disease, although the difference was deemed statistically insignificant. We could not establish a link between baseline uric acid levels and disease progression.
Our results show that increased levels of serum BMP7 levels are associated with the disease progression in AA renal amyloidosis CKD patients. Increased renal fibrosis may be main reason for disease progression.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Dirks JH, de Zeeuw D, Agarwal SK, et al. Prevention of chronic kidney and vascular disease: toward global health equity – the Bellagio 2004 Declaration. Kidney Int Suppl. 2005;98:S1–S6
- Lysaght MJ. Maintenance dialysis population dynamics: current trends and long-term implications. J Am Soc Nephrol. 2002;13:S37–S40
- Kronenberg F. Emerging risk factors and markers of chronic kidney disease progression. Nat Rev Nephrol. 2009;5:677–689
- Dember LM. Amyloidosis-associated kidney disease. J Am Soc Nephrol. 2006;17:3458
- Nguyen TQ, Goldschmeding R. Bone morphogenetic protein-7 and connective tissue growth factor: novel targets for treatment of renal fibrosis? Pharm Res. 2008;25(10):2416–2426
- Biyikli NK, Tugtepe H, Cakalagaoglu F, et al. Downregulation of the expression of bone morphogenetic protein 7 in experimental pyelonephritis. Pediatr Nephrol. 2005;20:1230–1236
- Zeisberg M, Bottiglio C, Kumar N, et al. Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am J Physiol Renal Physiol. 2003;285:F1060–F1067
- Duranay M, Segall L, Sen N, et al. Bone morphogenic protein-7 serum level decreases significantly in patients with contrast-induced nephropathy. Int Urol Nephrol 2011;43:807--812
- Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the modification of diet in renal disease study. Kidney Int. 1997;51(6):1908–1919
- Schwarz S, Trivedi BK, Kalantar-Zadeh K, et al. Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol. 2006;1(4):825–831
- Voormolen N, Noordzij M, Grootendorst DC, et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant. 2007;22:2909–2916
- Loghman-Adham M. Role of phosphate retention in the progression of renal failure. Lab Clin Med. 1993;122(1):16–26
- Cozzolino M, Staniforth ME, Liapis H, et al. Sevelamer hydrochloride attenuates kidney and cardiovascular calcifications in long-term experimental uremia. Kidney Int. 2003;64(5):1653–1661
- Obermayr RP, Temml C, Gutjahr G, et al. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19(12):2407–2413
- Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomerular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8):1887–1894