Abstract
Objective: The objective of this study is to evaluate the effect and mechanism of mitochondria-targeted peptides (MTP131 and SPI20) on contrast-induced acute kidney injury (CI-AKI) in rats with hypercholesterolemia. Method: Forty SD rats were randomly divided into normal diet group (NN, n = 8) and high cholesterol supplemented dietary group (HN, n = 32). At the end of 8 weeks, the group HN was divided into four subgroups. All Rats were given injection of either diatrizoate (10 mL/kg) or equal volume of normal saline, the rats pretreated with MTP131 or SPI20 were given injection with MTP131 or SPI 20 (3 mg/kg) by peritoneal cavity for 3 times. Blood, urine and renal tissue samples were prepared to determine biochemical parameters. The renal pathological changes were evaluated by hematoxylin and eosin staining and scored semiquantitatively, The protein expression of renal NOX4 was also measured by Western blotting. Results: In diatrizoate-injected rats, Serum creatinine (Scr), fractional excretion of sodium (FeNa%), fractional excretion of potassium (FeK%), pathological scores, renal malondialdehyde (MDA) content, the NADPH oxidase activity and the expression of NOX4 in kidney tissue were significantly increased (p < 0.01). In the groups pretreated with MTP131 or SPI20, the levels of Scr, FeNa%, FeK%, MDA content and NADPH oxidase activity in renal tissue decreased (p < 0.01), the levels of renal super oxygen dehydrogenises and ATPase activity increased (p < 0.01). The renal injuries induced by contrast media (CM) were alleviated. Conclusion: MTP131 and SPI20 might protect acute kidney injury induced by CM in rats with hypercholesterolemia.
Introduction
Contrast-induced acute kidney injury (CIAKI) is a serious complication of the use of iodinated contrast media (CM). At present, it is the third most important cause of acute renal failure in hospitalized patients, accounting for 12% of all cases and contributing to prolonged hospital stay and increased medical costs.Citation1 The present evidence indicates that the pathogenesis of CIAKI is mainly related to renal hypoxia, direct nephrotoxicity, and oxidative stress.Citation2 Oxidative stress seems to play an important role in CI-AKI. The optimal strategy to prevent CIAKI remains uncertain.
Many studies have shown that nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) was the major source of reactive oxygen species (ROS) in inflammation, hypertension, ischemic stroke, diabetic mellitus,Citation3 and NADPH oxidase also played an important role in the cisplatin or cyclosporine induced acute kidney injury (AKI).Citation4 Oxidative damage to mitochondria is a critical event in oxidative cell damage, and mitochondrial ROS should be a primary target for drug development.Citation5 A novel, water-soluble mitochondria-targeted peptide (MTP) was designed by Hazel H.Szeto and Peter W. Schiller, named MTP131 or SS-31,Citation6 and SPI 20 was another peptide in this series, which could scavenge ROS and inhibit lipid peroxidation in vitro. In addition, MTP131 and SPI 20 could reduce mitochondrial ROS, and prevent mitochondrial swelling. These peptides have demonstrated excellent efficacy in animal models of ischemia–reperfusion, neurodegeneration, and renal fibrosis.Citation7,Citation8 However, the protective effect of mitochondria-targeted peptides (MTP) on CIAKI was unclear. Therefore, the aim of this study was to explore the protective role of MTP on CIAKI in rats with hypercholesterolemia.
Materials and methods
Reagents and animals
The iodinated contrast medium was 76% diatrizoate meglumine (XinYi Pharmaceutical Co., Ltd, Shanghai, China), diluted to 300 mg I/mL with distilled water; The BCA Kit was purchased from Biyuntian Biotechnological Co., Ltd (Jiangsu, China); the NADPH oxidase Assay Kit was purchased from Jiemei gene medical technology Co., Ltd (Shanghai, China); MDA, SOD and ATPase Detection Kit were purchased from Jianchen Biotechnological Co., Ltd (Nanjin, China); NOX4 antibody of rabbit anti-mouse from Santa Cruz Biotechnological Co.,Ltd (USA), β-actin polyclonal antibodies of mouse anti-rat was purchased from Boao Biotechnological Co., Ltd (Beijing, China). The MTP (MTP131, SPI20) were provided by Stealth Peptides International Inc (Shanghai, China).
Forty healthy male SD rats weighing 180–220 g were provided by the Second Xiangya Hospital Animal Center. The rats were randomly divided into normal diet group (NN, n = 8) and high cholesterol supplemented dietary group (HN, 4% cholesterol and 1% cholic acid, n = 32).Citation9 At the end of 8 weeks, 1 rat given normal diet died and 4 rats given high cholesterol supplemented dietary died, then the rats with high cholesterol diet were randomly divided into four subgroups (7/group): high cholesterol diet group (HN), high cholesterol plus diatrizoate group (HH), high cholesterol plus diatrizoate plus MTP131 group (HM), and high cholesterol plus diatrizoate plus SPI20 group (HS). All experiments were approved by the Medical Science Animal Care Committee of the Central South University.
Experimental protocol
For the rats from group HH, HM and HS, 10 mL/kg b.w. of the CM (diatrizoate meglumine) with 300 mg I/mL were given through a caudal vein in 2 min to induce CIAKI;Citation9–12 at the same time, the other two groups were given equal volume of normal saline, 24 h before and after CM injection and half hour just before CM administration, the rats of group HM and HS were given injection of MTP131 or SPI 20 (3 mg/kg) into peritoneal cavity for 3 times, rats from group NN, HN, and HH were also given equal volume of normal saline. All rats were maintained in individual metabolic cages with free access to water to collect 24-h urine samples. Forty-eight hours before and after injection of CM, the urine samples were collected to determine urine creatinine, sodium and potassium. The blood samples were collected before and 48 hours after the administration of CM. Left kidney tissue was homogenated to measure the levels of malondialdehyde (MDA), superoxide dismutase (SOD), NADPH oxidase and adenosine triphosphatase (ATPase) activity according to the manufacturer’s instructions.
Serum and urinary biochemical indexes assay
Blood samples were collected for measurement of serum creatinine (Scr), potassium, sodium, cholesterol (CHOL), and triglyceride (TG). Urine was collected individually to measure urine creatinine, potassium, and sodium. Serum and urinary biochemical indexes were performed by an automatically biochemical analyzer (Beckmen synchron CX3, USA). Creatinine clearance (Ccr) was calculated by U × V/P where U = urine creatinine (mg/dL, V = urine volume (mL/min/100 g), and P = serum creatinine (mg/dL), and was expressed as mL/min/100 g body weight. Fractional excretion of sodium (FeNa%) and potassium (FeK%) were calculated as previously.Citation11
Measurements of renal MDA, SOD, ATPase and NADPH oxidase activity
In brief, 200 mg renal cortex was washed in ice-cold saline in tubes, cut into small pieces, and homogenized with ice-cold saline homogenization buffer at a ratio of 1:9 (w:v). The homogenate was centrifuged at 2000 rpm for 10 min at 4 °C. The supernatant was separated and analyzed. The MDA, SOD, ATPase and NADPH oxidase activities in the renal tissue were measured using commercial kits, according to the manufacturers’ protocol. The protein concentration of the homogenate was determined by the BCA assay method.
Renal histopathological examination
The right kidney tissue was fixed by 10% buffered formalin and embedded in paraffin. The kidney samples were cut to sections at four microns and stained by hematoxylin and eosin staining. Histological changes were observed and scored semiquantitatively in a blind manner using an arbitrary scale.Citation12 A minimum of 10 fields at 200 magnification were assessed and graded in renal cortex of each biopsy tissue. For tubular injury, the following score was used: 0 = no tubular injury; 1 = <25% of tubules injured; 2 = from 25% to 50% of tubules injured; 3 = from 51 to75% of tubules injured; 4 = more than 76% of tubules injured.
Western blotting for NOX4
Took freeze-stored crushed kidney tissues then join the ice-cold RIPA cracking fluid to extract total protein. Proteins (40 µg) were subjected to SDS-PAGE (12% acrylamide stacking gel and 10% running gel) and transferred onto nitrocellulose membranes. The membranes were then blocked with 5% skim milk in Tris-buffered Saline Tween (TBST) for 1 h at room temperature with the following incubation with a 1:500 dilution of a rabbit NOX4 antibody or a 1:1000 dilution of mouse polyclonal β-actin antibody at 4 °C overnight. The membranes were then washed with TBST (10 min × 3). Afterward, membranes were incubated with a HRP conjugated secondary antibodies (1:1000) for 1 hour at room temperature. Densitometric analysis of the membrane proteins was performed using a Kodak 4000 M Video Pro system.
Statistical analysis
The results were expressed as mean values ± standard deviation ( ± SD). Data were analyzed with SPSS 16.0 for windows program. Data were analyzed by one-way ANOVA, the two-groups comparison among multiple samples was carried out by LSD test. Pearson’s correlation was used. A p value less than 0.05 was considered statistically significant.
Results
Baseline characteristics of the rats
Before CM administration, no statistically significant difference was observed for the baseline characteristics of Scr, TG, Ccr, FeNa%, FeK% (p > 0.05). The serum CHOL value of high cholesterol diet rats was higher than those of normal diet rats (p < 0.05, ).
Table 1. Comparison of biochemical indicators in each group before contrast media injection ( ± SD, n = 7).
The effect of diatrizoate on Scr, Ccr, FeNa% and FeK%
In CM-administered rats, a trend toward higher value of Scr, FeNa% and FeK% accompanied by reduced Ccr value was observed when compared with control animals. The rats showed an evident increase in Scr, FeNa%, FeK% (p < 0.01) and a decline in Ccr (p < 0.01) after CM administration. However, in MTP-administered rats, pretreatment with MTP131 or SPI20 could significantly reduce the value of Scr, FeNa% and FeK% (p < 0.01), while the value of Ccr in MTP-administered rats was increased, but still lower than that in normal control animals ().
Table 2. Changes of renal function indicators in each group at 48 hrs after contrast media injection ( ± SD, n = 7).
Renal oxidative stress and ATPase parameters
As shown in , a decline in renal SOD and ATPase activity was observed in CM-injected rats, compared to control animals (p < 0.01), and this was accompanied by increased of renal MDA (p < 0.01). However, in MTP-administered rats, pretreatment with MTP131 or SPI20 could significantly reduce the values of MDA (p < 0.01), while the activity of SOD and ATPase were significantly increased (p < 0.01).
Table 3. Comparison of renal oxidative stress and ATPase parameters after contrast media injection ( ± SD, n = 7).
Renal NADPH oxidase activity and the expression of NOX4
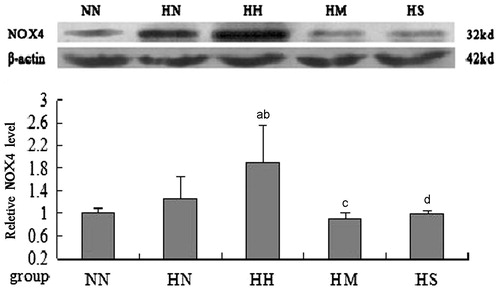
As shown in and , in CM-injected rats, the activity of renal NADPH oxidase and expression of NOX4 were significantly higher than those in control animals (p < 0.01); In MTP-administered rats, pretreatment with MTP131 or SPI20 could significantly reduce the activity of renal NADPH oxidase and expression of NOX4 (p < 0.01). And there was no significant difference of NADPH oxidase activity observed between normal diet and MTP-administered rats (p > 0.05, Table 3).
Renal histomorphology
As shown in and , in CM-injected rats, vacuolar degeneration of tubular epithelial cells, tubular dilation, protein cast, loss of tubular brush border, and increased epithelial cell shedding were observed, However, in MTP-injected rats, mild swelling of renal interstitium and vacuolar degeneration of focal tubular epithelial cells was observed. Semiquantitative analysis showed that the score of tubular injury in CM-injected rats were significantly higher compared to control animals (p < 0.01). Pretreatment with MTP131 or SPI20 could significantly reduce these lesions, the score of tubular injury in MTP-administered rats were significantly lower than that in CM-injected rats (p < 0.01).
Figure 1. Representative renal histomorphological changes: (A) NN, normal diet group; (B) HN, high cholesterol diet group; (C) HH, high cholesterol plus contrast media; (D) HM, high cholesterol plus diatrizoate plus MTP131 group; (E) HS: high cholesterol plus diatrizoate plus SPI20 group (HE staining, original magnification ×200).
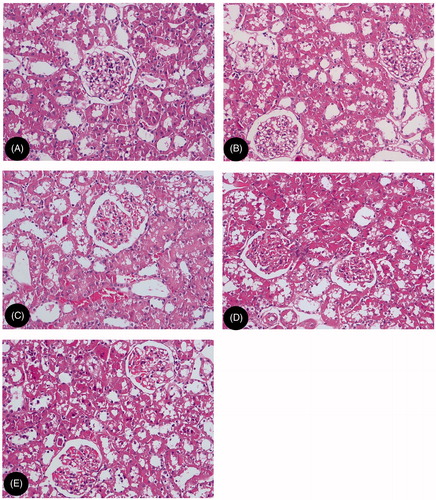
Figure 2. Comparison of tubular injury scores in each group after contrast media injection. Notes: NN, normal diet group; HN, high cholesterol diet group; HH, high cholesterol plus contrast media; HM, high cholesterol plus diatrizoate plus MTP131 group; HS, high cholesterol plus diatrizoate plus SPI20 group. ap < 0.01 versus NN group; bp < 0.01 versus HN group; cp < 0.01 versus HH group.
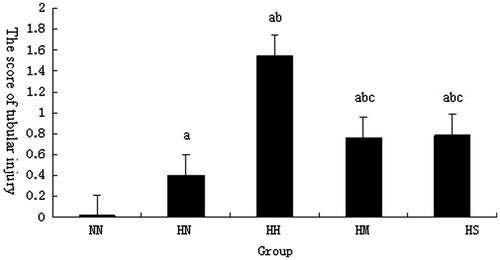
Figure 3. Protein expression of renal tissue NOX4 in each group (Western blotting, n = 3). Notes: NN, normal diet group; HN, high cholesterol diet group; HH, high cholesterol plus contrast media; HM, high cholesterol plus diatrizoate plus MTP131 group; HS: high cholesterol plus diatrizoate plus SPI20 group. ap < 0.05 versus NN group; bp < 0.05 versus HN group; cp < 0.05 versus HH group; dp < 0.01 versus HH group.
Correlation analysis
The study showed that the activities of MDA, and NADPH oxidase in renal cortex were positively correlated with tubular injury score in CM-injected rats (γ = 0.703, γ = 0.734; p < 0.05, respectively).
Discussion
The present study demonstrated that oxidative stress seems to play an important role in CI-AKI. MTP131 or SPI20 might protect acute kidney injury induced by CM in rats with hypercholesterolemia, which might be due to an antioxidant action, as well as increasing level of renal SOD and ATPase activity.
Many studies have shown that dietary hypercholesterolemia attenuates the endothelium-dependent relaxation of the renal artery.Citation13 Micropuncture measurements showed that hypercholesterolemia in rats’ induced marked vasoconstriction of renal blood vessels with a consequent fall in the glomerular filtration rate.Citation14 Under physiological conditions, these vascular effects are mainly functional, but if there is an additional insult, such as administration of CM, serious pathological injury may occur. Recently, Yang et al. found that CM administration increased serum creatinine levels and induced severe renal tubular necrosis in rats fed the high-cholesterol diet for 8 weeks but not in rats fed the normal diet or high-cholesterol diet for 2 weeks.Citation9 Our study also found that in CM-injected rats with hypercholesterolemia vacuolar degeneration of tubular epithelial cells, tubular dilation, protein cast, loss of tubular brush border, and increased epithelial cell shedding were observed. These studies demonstrated that hypercholesterolemia was an important risk factor for radiocontrast nephrotoxicity.
The recent research indicates that the pathogenesis of CIAKI is mainly related to renal hypoxia, direct nephrotoxicity, and oxidative stress.Citation2 NADPH oxidases represent a class of hetero-oligomeric enzymes whose primary function is the generation of ROS. It consists of seven members (Nox1-5, Duox1/2), each with a distinct cell and tissue distribution, only Nox4 protein has been identified in renal tubular cells abundantly.Citation15 NADPH oxidase played an important role in the cisplatin or cyclosporine induced acute kidney injury.Citation4 In this study, we observed that the Scr renal tissue MDA content, NADPH oxidase activity, the expression of NOX4, and the tubular injury score was significantly increased in diatrizoate-injected rats, while the Ccr, renal SOD and ATPase activity decreased significantly. It further supported that oxidative stress seems to play an important role in CI-AKI.
The optimal strategy to prevent CIAKI remains uncertain. There is no effective treatment for CIAKI in clinical work. The only current endorsed treatment is hydration.Citation16 Our previous studies showed amlodipine and telmisartan could protect the renal tissue from nephrotoxicity induced by diatrizoate in rat model.Citation17,Citation18 Another study indicated that enhanced formation of ROS in kidney after administration of CM play an important role in the development of CIAKI,Citation19 suggesting a protective effect of ROS scavenging or reducing ROS formation in CIAKI. Among these antioxidants, acetylcysteine (NAC) is the most studied agent for treatment of CIAKI. It is believed to reduce oxidative stress, which may be beneficial in preventing CIAKI.Citation20 The guidelines of Kidney Disease: Improving Global Outcomes suggest using oral NAC in patients to decrease the risk of CI-AKI.Citation21 However, there is increasing evidence suggesting that it is not efficacious in preventing CIAKI. And in the guidelines on contrast medium-induced nephropathy of CM Safety Committee, it is suggested that the efficacy of NAC in reducing the incidence of CIAKI remain unproven and their use cannot be recommended.Citation16
The MTP are a series of small peptides that are cell-permeable and selectively targeted and concentrate 1000–5000-fold in the inner mitochondrial membrane.Citation6 MTP131(SS31) and SPI20 are two forms of these peptides developed by Stealth Peptides International Company. They are small peptides limited to less than 10 amino acid residues. The structure of these peptides provide free radical scavenging abilities, Tyrosine-containing analogs can dose-dependently scavenge H2O2 OH− and ONOO−. By targeting the inner mitochondrial membrane, the MTP are ideally located to reduce mitochondrial oxidative stress. It was reported that mitochondria-targeted peptide MTP-131 could prevent both immortalized human trabecular meshwork and glaucomatous human trabecular meshwork cells from sustained oxidative stress induced by H(2)O(2).Citation22 Other studies have also shown that mitochondrial permeability transition, mitochondrial swelling, and cytochrome-C release could be significantly attenuated by addition of these peptides in vitro.Citation23 The animal studies suggested that these mitochondrial-targeted antioxidants may be very beneficial in the treatment of diseases models associated with oxidative stress.Citation24,Citation25 However, the effect of MTP on CIAKI is unclear. Our study found that pretreatment with MTP before diatrizoate infusion could reduce the Scr level, the renal contents of MDA, NADPH oxidase activity, the expression of NOX4 protein and the tubular injury score, while the levels of renal SOD and ATPase activity were increased. This indicated that MTP could protect the renal tissue from nephrotoxicity induced by CM.
In conclusion, our data showed that MTP (MTP131 and SPI20) could protect the renal tissue from nephrotoxicity induced by CM in rats with hypercholesterolemia, which might be due to an antioxidant action, as well as increasing level of renal SOD and ATPase activity.
Declaration of interest
The work was supported by grant from the Scientific Foundation Of Hunan Province, China (2010FJ6008, 2008JT3005).
Acknowledgements
We thank Stealth Peptides International Inc. (Shanghai, China) for providing us with MTP131 and SPI20.
References
- Itoh Y, Yano T, Sendo T, Oishi R. Clinical and experimental evidence for prevention of acute renal failure induced by radiographic contrast media. J Pharmacol Sci. 2005;97:473–488
- Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012;33:2007–2015
- Bylund J, Brown KL, Movitz C, Dahlgren C, Karlsson A. Intracellular generation of superoxide by the phagocyte NADPH oxidase: how, where, and what for? Free Radic Biol Med. 2010;49:1834–1845
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313
- Murphy MP, Smith RA. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv Drug Deliv Rev. 2000;41:235–250
- Rocha M, Hernandez-Mijares A, Garcia-Malpartida K, Banuls C, Bellod L, Victor VM. Mitochondria-targeted antioxidant peptides. Curr Pharm Des. 2010;16:3124–3131
- Kloner RA, Hale SL, Dai W, et al. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective Peptide. J Am Heart Assoc. 2012;1:e001644--e001657
- Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:601–619
- Yang D, Lin S, Wei L, Shang W. Effects of short- and long-term hypercholesterolemia on contrast-induced acute kidney injury. Am J Nephrol. 2012;35:80–89
- Andrade L, Campos SB, Seguro AC. Hypercholesterolemia aggravates radiocontrast nephrotoxicity: protective role of L-arginine. Kidney Int. 1998;53:1736–1742
- Yang DW, Jia RH, Yang DP, Ding GH, Huang CX. Dietary hypercholesterolemia aggravates contrast media-induced nephropathy. Chin Med J (Engl). 2004;117:542–546
- Duan SB, Liu GL, Chen GC, Wang P, Pan P, Xu XQ. Aged rats are susceptible to nephrotoxicity induced by iodinated contrast media. Ren Fail. 2013;35:150–154
- Stulak JM, Lerman A, Caccitolo JA, et al. Impaired renal vascular endothelial function in vitro in experimental hypercholesterolemia. Atherosclerosis. 2001;154:195–201
- Kaplan R, Aynedjian HS, Schlondorff D, Bank N. Renal vasoconstriction caused by short-term cholesterol feeding is corrected by thromboxane antagonist or probucol. J Clin Invest. 1990;86:1707–1714
- Krause KH. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn J Infect Dis. 2004;57:S28–S29
- Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527–2541
- Duan SB, Liu FY, Luo JA, et al. Nephrotoxicity of high- and low-osmolar contrast media. The protective role of amlodipine in a rat model. Acta Radiol. 2000;41:503–507
- Duan SB, Wang YH, Liu FY, et al. The protective role of telmisartan against nephrotoxicity induced by X-ray contrast media in rat model. Acta Radiol. 2009;50:754–759
- Quintavalle C, Brenca M, De Micco F, et al. In vivo and in vitro assessment of pathways involved in contrast media-induced renal cells apoptosis. Cell Death Dis. 2011;2:e155--e162
- Drager LF, Andrade L, Barros de Toledo JF, Laurindo FR, Machado Cesar LA, Seguro AC. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant. 2004;19:1803–1807
- Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204--218
- Chen M, Liu B, Gao Q, Zhuo Y, Ge J. Mitochondria-targeted peptide MTP-131 alleviates mitochondrial dysfunction and oxidative damage in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:7027–7037
- Zhao K, Zhao GM, Wu D, et al. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690
- Szeto HH, Liu S, Soong Y, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22:1041–1052
- Mizuguchi Y, Chen J, Seshan SV, Poppas DP, Szeto HH, Felsen D. A novel cell-permeable antioxidant peptide decreases renal tubular apoptosis and damage in unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2008;295:F1545–F1553
