Abstract
Aims: Stem cell transplantation for the treatment of kidney diseases is dependent on choice of transplant pathway. We evaluated the safety of human umbilical cord mesenchymal stem cells through peripheral infusion and their distribution in a rat model of renal interstitial fibrosis (RIF). Method: Cryopreserved umbilical cord mesenchymal stem cells were infused via tail vein injection into rats with unilateral ureteral obstruction and Sham-operated. Blood, kidney, heart, liver, spleen and lung were collected at 14, 21, and 28 days after infusion. Testing included microscopic observation of kidney morphological changes and immunohistochemical testing to identify and count the number of MAB1281 (labeled human cells) positive cells in the heart, liver, spleen, lungs, and kidneys of different treatment groups. Results: There was no significant difference in the Sham-operated group and Sham-operated + cell transplantation group at different time points. Human cells were identified mainly in the lungs, spleen, and kidney. The number of human umbilical cord mesenchymal stem cells in the kidney was greater in the unilateral ureteral obstruction + cell transplantation group, compared to the Sham-operated + cell transplantation group. human umbilical cord mesenchymal stem cells were mainly located in the interstitium of the left kidney. These results suggest that infused mesenchymal stem cells were primed to engraft a damaged kidney, especially damaged renal interstitium. Conclusions: Intravenous infusion of exogenous umbilical cord mesenchymal stem cells is feasible and safe. Infused mesenchymal stem cells can reach damaged kidney tissues with obstructive RIF after a vein graft.
Introduction
Chronic progressive renal interstitial fibrosis (RIF) is a common pathway toward end-stage renal failure. It is important to prevent or reverse RIF as early as possible in order to delay or prevent end-stage renal failure. With the development of regenerative medicine, increasing number of reports described the treatment of kidney diseases with stem cell transplantation (SCT). Many researchers are exploring the optimal transplantation methods, that is the means by which transplanted stem cells for better migration into kidney.
Kidneys are retroperitoneal organs with a rich blood supply. Stem cells are generally transplanted into kidneys via renal artery infusionCitation1, peripheral intravenous infusionCitation2 or renal subcapsular injection. Renal artery infusion and renal subcapsular injection can be invasive and can cause additional local inflammatory responses.Citation3,Citation4 The invasive techniques are theoretically more effective in terms of local delivery of stem cells. In contrast, transplantation by peripheral intravenous infusion is relatively simple to perform in the clinic and less invasive. It is more feasible in clinical treatment and used by the majority of researchers. It is not known if intravenously infused mesenchymal stem cells (MSC) reach the kidneys. Numerous studiesCitation5–8 have demonstrated that MSC can target the site of real injury. Herrera MBCitation9 intravenously infused bone marrow MSC in a mouse model of acute renal failure caused by intramuscular injection of glycerol. Transplanted stem cells were found in the kidneys, mainly in the tubular cortex. Kunter UCitation10 performed bone marrow MSC tail vein injections in a rat model of mesangial proliferative glomerulonephritis. Transplanted fluorescent (positive) cells were found in the kidney within 6 days of injection. These results suggest that peripheral venous infusion is an effective method of cell therapy in renal diseases. It is not known how many of the injected MSC home to damaged renal tissues. Some reports have demonstrated whole body distribution of transplanted bone marrow MSC in mice after radiationCitation11 and in the mouse model of immuno-deficiency.Citation12 Injected MSC were found preferentially in injured tissues as well as lung, bone marrow, spleen, skin, and kidney.
MSC derived from umbilical cord blood or umbilical cord cells are being used to treat renal disease in animals. There are no reports describing the distribution of intravenously transplanted umbilical cord mesenchymal stem cells (UC-MSC) in the organs of animals used in renal injury models. We used a rat model of RIF (caused by unilateral ureteral obstruction, UUO) to study the transplantation of human UC-MSC after tail vein injection. The treatment was safe and cells were delivered to the kidneys. We examined the feasibility of treating RIF by UC-MSC transplantation. Our results provide a theoretical and experimental base for the clinical treatment of chronic kidney disease.
Materials and methods
Cell culture, recovery, and expansion
Umbilical cords were freshly collected under sterile conditions. Adherent cells were separated by sequential digestion using combined enzymes. Cells were isolated, cultured, and expanded. MSC were identified by cell morphology, cell surface antigen expression, and induced osteogenic and adipogenic differentiation. UC-MSCs from passage 5 were prepared as 5 × 106/mL in PBS.
Experimental groups and sample collection
Eight-week-old male SD rats (n = 84) were divided into 4 groups by a random number table. (1) Unilateral ureteral obstruction group (UUO group, n = 21): the tail vein was injected with 1 mL PBS 7 days after left ureteral ligation. (2) Unilateral ureteral obstruction + cell transplantation group (UUO + MSC group, n = 21): the tail vein was injected with 1 mL UC-MSC 7 days after left ureteral ligation. (3) Sham-operated group (Sham group, n = 21): the same surgical approach was used as in the UUO group. The left ureter was freed but not ligated. The tail vein was injected with 1 mL UC-MSC 7 days after surgery. (4) Sham-operated + cell transplantation group (Sham + MSC group, n = 21): the same surgical approach was used as in the Sham group. The tail vein was injected with 1 mL UC-MSC 7 days after surgery.
Blood, kidney, heart, liver, spleen, and lung tissue was collected from 7 animals in each treatment group at 14, 21, and 28 days after infusion. Tissues were fixed in formalin for 8 to 12 h after dehydration, cleared in xylene, penetrated in wax and embedded in paraffin. Sections were examined using light microscopy and immunohistochemical staining.
Observation and experimental methods
Experimental animals
General information and body weight before surgery and prior to sacrifice were recorded. Blood count and serum alanine aminotransferase and serum aspartate aminotransferase levels were determined at different time points.
Renal morphologic changes at different time points were observed using light microscopy
Kidney tissue blocks were cut into 4 μm sections. Hematoxylin & Eosin (HE) staining, periodic acid-Schiff reaction (PAS) staining and Masson staining were performed on sections for observation of kidney morphological changes.
Detection of MAB1281 positive cells in animal tissues
The StreptAvidin-Biotin Complex (SABC) method was used. Sections of rat heart, liver, spleen, lung, and kidney (4 μm) were deparaffinized, blocked with normal sheep serum after antigen retrieval, and digested by microwave. Dilute mouse anti-human monoclonal antibody MAB1281 (Millipore, Billerica, MA) was added and incubated at 4 °C overnight and then 1 h at room temperature. Sections were incubated with biotin labeled sheep anti-mouse secondary antibody (Boster, China) at room temperature for 20 min, developed with 3,3′-Diaminobenzidine (DAB), counterstained with hematoxylin, and then mounted.
MAB1281 specifically reacts with the nucleus of all human cells. This method was used for the identification of HU-MSC. Ten nonoverlapping fields were randomly examined under high magnification (×200). The positive cells were brown or yellow-brown. Positive cells were counted and mean values were calculated. ImagePro + 6.0 image analysis software was used.
Statistical analysis
The SPSS13.0 statistical software program was used for statistical analysis. Data from each group was presented as mean ± standard deviation (±SD). Two sets of data were compared using the Student’s T-test. Multiple sets of data with homogeneity of variance were compared using One-way ANOVA analysis. The Fishers Least Significant Difference (LSD) test was used to compare the mean of one group with the mean of another. Data with heterogeneity of variance were converted to homogeneity and then analyzed. α = 0.05 was a standard. p < 0.05 was considered statistically significant.
Results
Experimental animals
In each group, the body weight of the rat increased with age. The body weight gain in UUO group and UUO + MSC group was less than that in the Sham group and the Sham + MSC group (p < 0.01). There was no significant difference (p > 0.05) in body weight gain between the UUO and UUO + MSC groups, and between the Sham and Sham + MSC groups. There were no grossly obvious visual abnormalities in the heart, liver, spleen, and lungs of animals in the four groups.
Changes in blood count and liver function at different time points in the four groups
No significant differences were found in white blood cells, hemoglobin, platelets, and serum glutamic pyruvic transaminase among the four groups, at three time points. Serum aspartate aminotransferase levels were significantly higher in the UUO + MSC group compared to the Sham group and Sham + MSC group (p < 0.01), and were significantly lower than in the UUO group (p < 0.01).
Morphological changes in renal tissue at different time points
No significant changes in kidney tissues were observed in the Sham group. Renal tissues in the Sham + MSC group showed no obvious inflammatory cell infiltration after cellular infusion. The contralateral renal tissues (right kidney) from both UUO and UUO + MSC groups also showed no significant changes after treatment. The kidney corresponding to the ligated ureter (left kidney) gradually increased in size after obstruction. There was renal pelvis fluid expansion and gradual thinning of the renal parenchyma in UUO group and UUO + MSC group. There was renal tubular epithelial cell atrophy, loss of partial of the small tube, cystic expansion of the distal tubules and collecting ducts, infiltration of inflammatory cells, cellular proliferation, and fibrosis. There were no obvious glomerular lesions. Pathological changes in the left kidney in the UUO + MSC group was less severe than in the UUO group at the three time points.
Immunohistochemical staining
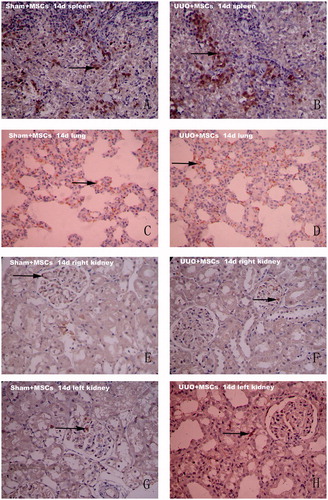
MAB1281 positive staining was used as a marker for exogenous UC-MSC. No MAB1281 positive cells were observed in the heart, liver, spleen, lung, or kidney in both Sham and UUO groups. The PBS negative control group showed no UC-MSC. The number of MAB1281 positive cells in both the Sham + MSC group and the UUO + MSC group was different at the different time points. MAB1281 positive cells were mainly distributed in the lungs and spleen. Fewer cells were present in the kidney, heart and liver. There was a decreasing trend in the number of positive cells (). There were significantly more MAB1281 positive cells in spleens from the Sham + MSC group than from the UUO + MSC group at 14 days after surgery (p < 0.01). There was no difference in MAB1281 positive cells in lungs from the two groups (p > 0.05). MAB1281 positive cells were mainly distributed in the glomeruli of the right kidney in both groups and in the glomeruli of the left kidney in the Sham + MSC group. MAB1281 positive cells were most common in the interstitium of the left kidney (see ) in the UUO + MSC group. No MAB1281 positive cells were seen in the right kidneys of both groups at 21 or 28 days after surgery. There was no difference in the number of MAB1281 positive cells in the spleen or lungs in the two groups (p > 0.05).
Figure 1. The number of MAB1281 positive cells in the spleen, lung and left kidney change over time in the Sham + MSC group (left) and UUO + MSC group (right).
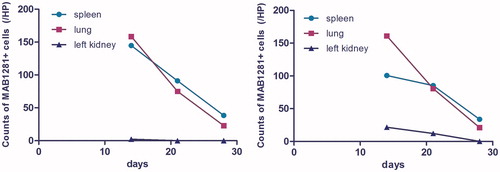
Figure 2. Immunohistochemical staining results (×200). MAB1281 positive cells in the spleen, lung, right kidney and left kidney in both groups at day 14 after surgery. Sham + MSC group spleens (A) and lungs (C), UUO + MSC group spleens (B) and lungs (D) all showed a large number of positively staining brown cells; (E): In Sham + MSC group, the right kidney occasionally showed positively staining brown cells; (F): In UUO + MSC group, the right kidney glomeruli occasionally showed positively staining brown cells; (G): In Sham + MSC group, the left kidney occasionally positively staining brown cells in the glomeruli; (H): In UUO + MSC group, the left kidney showed a small amount of positively staining brown cells. Most positive cells were in the renal interstitium. Arrows indicate MAB1281 positive cells.
Discussion
The safety of intravenous infusion of umbilical cord mesenchymal stem cells
MSC are stem cells derived from mesoderm and have the capacity for self-renewal. They are multipotent and give rise to different cell lineages. UC-MSCs have several advantages over MSC derived from bone marrow. They are more easily isolated, they are obtained in a non-invasive fashion, and they are pathogen free. UC-MSCs are thought to be a better source of MSC. Animal studies have shown that UC-MSC are low in immunogenicity and can evade recognition by immune cells. UC-MSCs are well tolerated after infusion, even if there is not a MHC match.Citation13 The low immunogenicity of UC-MSC may be due to their moderate level of expression of MHCI molecules and low level of expression of MHCII molecules.Citation14 The number of sources of UC-MSC in the body is limited and it is hard to meet the needs of clinical therapy. Allogeneic UC-MSC transplantation in the clinic seems to have broad prospects.
Human UC-MSCs have low immunogenicity and immunomodulatory and immunosuppressive functions that serve to alleviate acute immune rejection.Citation15 The cells are easy to isolate, they are highly proliferative, and there are no moral objections to their harvest. All these advantages make allogeneic MSC a promising source for clinical application.Citation16
Human UC-MSC used in animal experiments must be evaluated for immune rejection caused by the xenograft. We studied the safety of UC-MSC xenografts by injection of 5 × 106/mL PBS UC-MSC into the tail veins of UUO + Sham rats. We found that all rats survived after infusion (except one rat died before left ureteral ligation because of anesthetic intolerance). There was no difference in body weight gain, routine blood tests and serum alanine aminotransferase levels in the Sham and Sham + MSC groups and in both UUO + MSC groups. Serum aspartate aminotransferase levels in the UUO + MSC group were higher than normal, but less than those in the UUO group. This suggests that the increase in aspartate aminotransferase may benefit cell transplantation. Significant inflammatory cell infiltration and inflammatory response was not seen in the heart, liver, spleen, lungs, or kidneys in the Sham + MSC group on histopathologic exam. We think it is safe and feasible to intravenously transplant human UC-MSC into rats since it did not cause changes in biochemical parameters or histopathological lesions in organs.
Distribution of umbilical cord mesenchymal stem cells in rats with obstructive renal interstitial fibrosis
It is not known if peripherally infused cells can reach the kidneys. In order to track the distribution of cells after infusion, a suitable marker must be identified. Commonly used markers are green fluorescent protein (GFP), 4′,6-diamidino-2-phenyl-indole (-DAPI), PKH26 fluorescent dyeCitation10, and superparamagnetic iron oxide (SPIO).Citation9,Citation17 There are no well-established methods to label human cells. In this study, we infused human cells into rats. We chose mouse anti-human nuclear monoclonal antibody as the primary antibody to track human UC-MSC in the rat, using immunohistochemical methods.
We found that human UC-MSC were mainly distributed in the lungs and spleen, and less so in the kidney, myocardium, and liver. More UC-MSC were seen in the lungs of both groups at 14 days after surgery. This may be because MSC are larger, less flexible than hematopoietic cells, and easily trapped in the pulmonary circulation after injection. We found that the number of UC-MSC in the lungs significantly decreased over time. The number of MSC in the lungs 3 weeks after infusion was only one-eighth the number 1 week after infusion, This suggests that human UC-MSC in the lung may reenter the blood circulation or are cleared by the body. There are no literature reports describing MSC distribution after venous infusion. In contrast to the findings in the lungs, the number of UC-MSC in spleens from the UUO + MSC group was significantly less than in the Sham + MSC group. The number of UC-MSC in kidneys from the UUO + MSC group were significantly greater than in the Sham + MSC group. UC-MSC were mainly found in renal interstitium of the left kidney (injured kidney) in the UUO + MSC group. UC-MSC were mainly found in glomeruli of the right kidney of the UUO + MSC group and the normal kidney of the Sham + MSC group. MSC in the UUO + MSC group, may be able to detect injured tissues and migrate to them. We found no MAB1281 positive cells in kidneys from the UUO + MSC and Sham + MSC groups at 28 days after surgery. This may be due to the lower number of cells in the kidney, cell death, or cells cleared out by local immune mechanisms.
In summary, it is unknown how infused cells travel to injured tissues and what their long-term behavior is in different organs. We found that infused UC-MSC can reach injured rat renal tissues with obstructive RIF.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Asanuma H, Vanderbrink BA, Campbell MT, et al. Arterially delivered mesenchymal stem cells prevent obstruction-induced renal fibrosis. J Surg Res. 2011;168:e51–e59
- Koseki C, Herzlinger D, al-Awqati Q. Integration of embryonic nephrogenic cells carrying a reporter gene into functioning nephrons. Am J Physiol. 1991;261:C550–C554
- Cao H, Qian H, Xu W, et al. Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol Lett. 2010;32:725–732
- Aoi T, Yae K, Nakagawa M, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702
- Bittner RE, Schofer C, Weipoltshammer K, et al. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol (Berl). 1999;199:391–396
- Eglitis MA, Dawson D, Park KW, Mouradian MM. Targeting of marrow-derived astrocytes to the ischemic brain. Neuroreport. 1999;10:1289–1292
- Mansilla E, Marin GH, Sturla F, et al. Human mesenchymal stem cells are tolerized by mice and improve skin and spinal cord injuries. Transplant Proc. 2005;37:292–294
- Xiang GA, Zhang GQ, Fang CH, Gao P, Chen KY. A preliminary study of the homing capacity of allograft mesenchymal stem cells to rat liver. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:994–997
- Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–1041
- Kunter U, Rong S, Djuric Z, et al. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006;17:2202–2212
- Francois S, Bensidhoum M, Mouiseddine M, et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24:1020–1029
- Bentzon JF, Stenderup K, Hansen FD, et al. Tissue distribution and engraftment of human mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene. Biochem Biophys Res Commun. 2005;330:633–640
- Maitra B, Szekely E, Gjini K, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604
- Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001
- Zhang W, Qin C, Zhou ZM. Mesenchymal stem cells modulate immune responses combined with cyclosporine in a rat renal transplantation model. Transplant Proc. 2007;39:3404–3408
- Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Hematologica. 2006;91(8):1017–1026
- Yoo JH, Park C, Jung DI, et al. In vivo cell tracking of canine allogenic mesenchymal stem cells administration via renal arterial catheterization and physiopathological effects on the kidney in two healthy dogs. J Vet Med Sci. 2011;73:269–274
