Abstract
Purpose: In this study, it is aimed to compare the serum leptin and PAI-1 levels and evaluate their relationship in children on hemodialysis (HD) and peritoneal dialysis (PD). Method: Thirty-six patients on HD (mean age: 15.0 ± 2.8 years), 19 patients on PD (mean age: 13.0 ± 3.5 years) and 15 healthy subjects (mean age: 14.5 ± 2.7 years) were included in the study. Laboratory investigations included blood count, biochemical parameters, serum iron, iron binding capacity, parathormone, erythrocyte sedimentation rate, C-reactive protein (CRP), prothrombin time (PT), partial thromboplastin time (PTT), fibrinogen, serum leptin and PAI-1 levels. Results: Serum leptin levels were significantly higher in HD group than in control group when the effects of BMI and sex were controlled, while PD and control groups had similar leptin levels. PAI-1 levels were also significantly higher in HD group than in control group, while there was no statistically significant difference in PAI-1 levels of PD and control group. PAI-1 levels and leptin levels were significantly correlated, which was independent of the effect of BMI in both HD and PD groups when they are evaluated separately. Conclusion: Results of our study showed that HD patients had higher leptin and PAI-1 levels and leptin and PAI-1 levels were correlated significantly in both patient groups. The effect of elevated serum leptin and PAI-1 levels on the cardiovascular complications remains to be established.
Introduction
Cardiovascular complications are among of the major causes of mortality in adults and 25% of mortality in children with end stage renal disease (ESRD).Citation1,Citation2 In recent years, the well-known relation between obesity and cardiovascular mortality has been evaluated on the basis of insulin resistance and dyslipidemia.Citation3 Especially, definition of functions of hormone “leptin” has resulted in a variety of studies. Leptin is secreted from adipocytes and has a key role in the regulation of body weight.Citation3 Its plasma concentration is elevated in chronic renal disease. Similarly, it is known that disorders of fibrinolytic system are important in the development of atherosclerosis and cardiovascular disease (CVD).Citation4,Citation5 Plasminogen activator inhibitor-1 (PAI-1) is the inhibitor of plasmin which is the key enzyme in fibrinolytic system. Elevation in PAI-1 has been suggested to be a risk factor for CVD.Citation5
Studies on general population and other disease groups have shown that leptin and PAI-1 levels are related.Citation6,Citation7 On the basis of data summarized above, the relationship between leptin and PAI-1 levels in patients with ESRD can have a role in CVD. Review of the literature revealed no study evaluating the leptin and PAI-1 levels together in children with ESRD. In this study, we aimed to evaluate the relationship between serum leptin and PAI-1 levels and compare their levels in hemodialysis (HD) and peritoneal dialysis (PD) groups.
Materials and methods
Thirty-six patients (19 female, 17 male) on HD, 19 patients (10 female, 9 male) on PD and 15 healthy subjects (9 female, 6 male) were included in the study. The inclusion criteria for the study were normal liver function, no recent sign of inflammation or known thrombosis, and no blood transfusion within the last month. The patients on HD underwent 4 h of treatment 3 times weekly and were dialyzed using a hemophage membrane. All these children were dialyzed via arteriovenous fistulae and with bicarbonate-buffered solution. The patients on PD were dialyzed with 1.36–3.86% glucose containing dialysate.
The age, the disease causing ESRD, the time period from the start of chronic dialysis, drugs used, dose of erythropoietin, height and weight of the subjects were recorded. The weekly erythropoietin dose, body mass index (BMI) and total cholesterol/HDL ratio of the subjects were calculated.
Blood samples were withdrawn in 08:00 am after 8 h of night-time fasting, before the dialysis section in HD patients and during routine visits in PD patients and control subjects. Peripheral venous blood samples for PAI-1 levels were obtained without venous stasis, by using 21 G needles and put into tubes containing 0.3 mL 3.8% sodium citrate. After sampling, platelet free plasma was obtained by centrifuging at 1000 rpm for 15 min and stored at −20 °C until the analysis.
Laboratory investigations included blood count, biochemical parameters, serum iron, iron binding capacity, ferritin, erythrocyte sedimentation rate, C- reactive protein (CRP), prothrombin time (PT), partial thromboplastin time (PTT), fibrinogen, serum leptin and PAI-1 levels.
The study was approved by the local ethical committee (Baskent University Research Committee Project No: KA03/76, 2003. Informed consent was taken for each patient.
Leptin was measured by radioimmunoassay (RIA) using a commercially available kit (Linco Research, Inc., St. Charles, MO). PAI-1 was measured by colorimetric assay using the commercially available kit (Chemi-Con International, Inc., Cat No. ECM610, Temecula, CA).
Logarithmic transformation was performed for the data related to leptin since they were not normally distributed. One-way analysis of variance was used to compare the group findings of continuous variables. Pearson’s and Sperman correlation coefficients were used to evaluate the presence and degree of correlations according to the features of variables. Analysis of co-variance was used to exclude the effect of other variables on the specific variable while comparing the group means. The relation between one dependent variable and two independent variables was evaluated by using multiple regression analysis. All statistical analysis was performed by using the soft ware “SPSS 11.0 for Windows”. Probability values less than 0.05 were considered significant.
Results
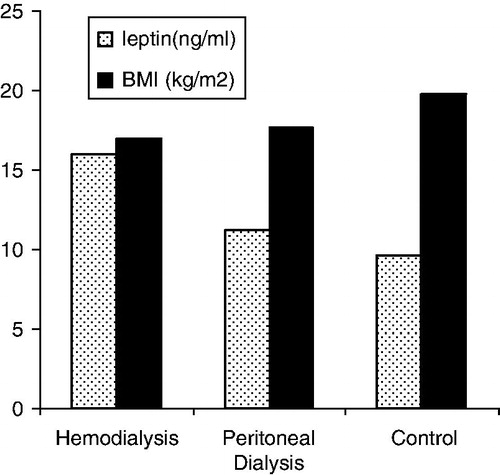
A total of 70 subjects were included in the study. The age, sex, BMI and some other findings of the groups are summarized in . The mean age was 15.0 ± 2.8 years in HD, 13.0 ± 3.5 years in PD and 14.5 ± 2.7 years in control group (p > 0.05). The groups were not different with respect to age and sex distribution (p > 0.05). The mean body weight, height and body mass index (BMI) of HD and PD group were similar (p > 0.05), but control group had a significantly higher mean body weight, height and BMI than HD and PD group (p < 0.05). There was no difference in the time period from the start of chronic dialysis between HD and PD groups (p > 0.05). Hematological findings of the subjects are summarized in . The mean hemoglobin, hematocrit and thrombocyte levels were lower in HD and PD group than the controls. The mean ferritin levels were higher in HD and PD patients than the controls. The mean PT and PTT levels were not different among three groups (p > 0.05). Biochemical parameters and acute phase reactants are given in . The mean total cholesterol level was higher in HD than in PD and control group (p < 0.05). The mean HDL, VLDL and triglyceride levels were similar in HD and PD groups (p > 0.05), but they were higher than control group (p < 0.05). The mean total protein and albumin levels were not different in HD and control group, but they were found to be significantly lower in PD group than other groups (p < 0.05).
Table 1. Demographic and anthropometric findings of study groups.
Table 2. Hematological findings of subjects.
Table 3. Biochemical parameters and acute phase reactants of subjects.
Erythrocyte sedimentation rate, CRP and fibrinogen levels were higher in HD and PD group compared to control group (p < 0.05).
Serum leptin and PAI-1 levels
The mean absolute levels of serum leptin in HD, PD and control groups are shown in . Multiple regression analysis showed that the mean log leptin levels were significantly higher in HD group than in control group when the effects of BMI and sex were controlled (r2 = 0.523, adjusted r2 = 0.491, p < 0.05), while there was no statistically significant difference between the mean log leptin levels of PD and control group (r2 = 0.713 and adjusted r2 = 0.681, p > 0.05).
When the patients are grouped according to total protein level (cut-off level of 6 g/dL) and albumin levels (cut-off level of 4 g/dL), the median serum leptin level was higher in both patient with total protein level higher than 6 g/dL and albumin level higher than 4 g/dL (p < 0.05). In analysis of co-variance, BMI was added to the model to eliminate its effect, the difference in serum leptin level according to serum albumin and total protein level persisted (r2:0.471, adjusted r2:0.454 and r2:0.514, adjusted r2:0.599, respectively, p < 0.05).
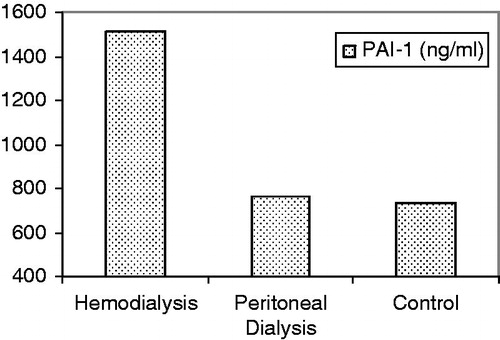
The mean PAI-1 levels were 1583 ± 206, 791 ± 220 and 716 ± 253 ng/mL in HD, PD and control groups, respectively. The mean PAI-1 level of HD group was significantly higher than those of control group (p < 0.05), while PD and control groups had similar mean PAI-1 levels (p > 0.05; ).
Correlations
The correlations between serum leptin, PAI-1 levels and other parameters are summarized in .
Table 4. The correlations between log leptin, PAI-1 and age, BMI, dose of erythropoetin, nutritional markers, inflammatory findings and cardiovascular risk factors in the group of patients on hemodialysis and peritoneal dialysis (n = 55).
PAI-1 levels were correlated with log leptin levels, which was independent of the effect of BMI in whole group of subjects (r2 = 0.353, adjusted r2 = 0.321, p < 0.0001). After the addition of BMI and sex to the model of linear regression analysis to control their effect, this positive correlation persisted (r2 = 0.353, r2 adjusted = 0.321, p < 0.0001).
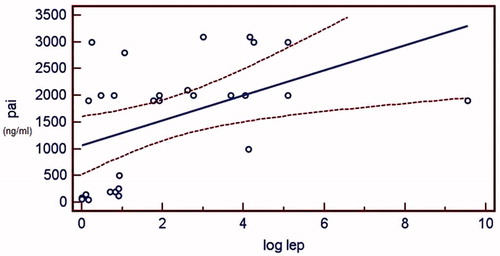
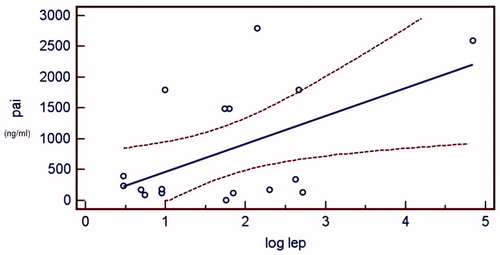
The correlation between PAI-1 and log leptin was also examined in HD and PD groups separately. Both in HD and PD group, log leptin and PAI-1 were significantly correlated and the correlation persisted after the effect of BMI was eliminated (r2 = 0.650, adjusted r2 = 0.621 in HD group and r2 = 0.679, adjusted r2 = 0.636 in PD group, p < 0.05). The and demonstrate the regression plots of log leptin and PAI-1 in HD and PD groups, respectively.
There was a significant correlation between log leptin and serum total protein and albumin levels (r = 0.369 vs. r = 0.343, respectively, p < 0.05). The correlation persisted after the effect of BMI was controlled (r2 = 0.706, r2 adjusted = 0.498, p < 0.05).
Log leptin was significantly correlated with serum total cholesterol/HDL ratio and LDL levels (r = 0.260 and r = 0.283, respectively, p < 0.05). Also Serum PAI-1 level was correlated with total cholesterol/HDL ratio and LDL levels (r = 0.259 and r = 0.296, respectively, p < 0.05).
PAI-1 levels were correlated with weekly erythropoietin dose and erythropoietin/hematocrit ratio (r = 0.464 and r = 0.439, respectively, p < 0.05).
Discussion
Since the kidneys are known to play role in the clearance of many polypeptide hormones like parathyroid hormone and glucagon, leptin is expected to accumulate in renal failure. However, increased leptin level is not a constant finding in patients with ESRD and etiology of increased leptin levels in uremia has not been well understood. Serum leptin levels were higher in patients with the signs of ongoing inflammation.Citation8 Daschner et al.Citation9 reported that the percentage of body fat remains the main determinant of serum leptin in children with ESRD but their levels increase with declining glomerular filtration. In our study, serum leptin level was found to be higher in HD group after the effect of BMI and sex were eliminated. Review of the literature revealed some studies with a variety of results, some reporting higher leptin levels in HD, some reporting contrary results.Citation10–17 In different studies, variety in BMI of patients and other factors affecting the protein binding of leptin can affect leptin levels both in HD and PD patients. More stabile and continuous clearance of free leptin in PD patients can contribute to lower serum leptin levels in PD patients.Citation18 Also transperitoneal protein loss causing hypoalbuminemia in PD patients can contribute to lower serum leptin levels in PD patients.
Thus, nutritional condition, BMI, sex of the patients, type of filters used and transperitoneal protein loss are the factors affecting serum leptin levels of patients on dialysis. Our results showed higher serum leptin levels in HD patients suggesting that HD patients are exposed more intensively to negative effects of hyperleptinemia.
Human PAI-1 is a single chain glycoprotein with a molecular weight of 43 kDa. Its rapid interaction with tissue plasminogen activator (tPA) may function as a major control point in the regulation of fibrinolysis. High concentrations of PAI-1 have been associated with thromboembolic disease and increased cardiovascular mortality.Citation5
In our study, PAI-1 levels were found to be higher in HD group than in control while PD group had similar PAI-1 levels to control group. Review of literature revealed different results. Irish et al.Citation19 and Tamura et al.Citation2 reported lower PAI-1 levels in HD patients than PD and controls. However, many studies have recently demonstrated that endothelial cell related glycoproteins including PAI-1 are increased in HD patients.Citation14,Citation16,Citation20–25 These findings are thought to be related to subclinical endothelial injury in ESRD patients.Citation2,Citation21 However, there are other factors contributing to higher PAI-1 levels. Chronic inflammation is one of these factors. It has been suggested that recurrent acute phase reaction as a result of cytokine activation due to contact with dialysis membranes can cause increase in PAI-1 as an acute phase reactant.Citation19,Citation20 PAI-1 has been shown to be correlated with other acute phase reactants like CRP and IL-6, supporting this hypothesis. Results of our study showed higher ESR, CRP, fibrinogen and ferritin levels in ESRD patients than the controls. However, no significant correlation was detected between PAI-1 levels and these acute reactants.
The most striking finding of our study is the positive correlation between serum PAI-1 and leptin levels. Malyzsko et al.Citation18 demonstrated that leptin was correlated with tissue factor pathway inhibitor, protein C, thrombomodulin and ristocetin induced thrombocyte aggregation. Review of the literature revealed no study evaluating the relation between leptin and PAI-1 in children. Although correlations do not necessarily mean a biological cause-result relationship, we demonstrated increase in leptin levels parallel PAI-1 levels. Leptin is almost completely synthesized in adipose tissue. PAI-1 is known to be synthesized by hepatocytes and endothelial cells but it has been also shown to be synthesized in adipose tissue.Citation7 These findings may bring into mind that the parallel increase in PAI-1 and leptin levels are related to body fat mass. However, we showed that the significant correlation between PAI-1 and leptin is independent of BMI. This finding is compatible with the findings of De Mitrio et al.Citation7 which showed that PAI-1 levels were correlated with leptin levels independent of BMI in premenopausal women. These findings suggest that leptin may increase the synthesis of PAI-1 by a direct and independent effect. Further study is needed to establish the hypothesis of possible direct effect of leptin on PAI-1 synthesis. The relationship between leptin and PAI-1 levels suggest that changes in leptin level by its effect on PAI-1, may contribute to tendency to thrombosis and related complications and long-term CVD risk in ESRD patients.
Recombinant human erythropoietin is effective in correcting renal anemia. However, erythropoietin replacement therapy is associated with increased risk of thrombosis. Transient changes in protein C and thrombin-antithrombin III complex during erythropoietin treatment have been demonstrated and may represent markers of prothrombotic tendency.Citation25–27 In our study, we demonstrated positive correlation between PAI-1 level and erythropoietin dose/hematocrit ratio. This finding was consistent with previous reports, indicating that erythropoietin therapy suppresses fibrinolytic capacity increasing thrombotic tendency.
In conclusion, the results of our study revealed that serum leptin and PAI-1 levels are higher in HD patients and they are correlated independent of the effect of BMI and sex in both HD and PD patients. The effect of elevated serum leptin and PAI-1 levels on the development of cardiovascular complications in children on chronic dialysis remains to be established.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Parekh RS, Gidding SS. Cardiovascular complications in pediatric end stage renal disease. Ped Nephrol. 2005;20:125–131
- Tomura S, Nakamura Y, Doi M, et al. Fibrinogen, coagulation factor VII, tissue plasminogen activator, plasminogen activator inhibitor-1, and lipid as cardiovascular risk factors in chronic hemodialysis and continuous ambulatory peritoneal dialysis patients. Am J Kidney Dis. 1996;27:848–854
- Cameron AJ, Dunstan DW, Owen N, Zimmet PZ, Barr EL, Taksin AM. Health and mortality consequences of abdominal obesity: evidence from the AusDiab study. Med J Aust. 2009;191:202–208
- Hamsten A, Wiman B, de Faire U, Blomback M. Increased plasma leptin levels of a rapid inhibitor of tissue plasminogen activator in young survivor of myocardial infarction. N Eng J Med. 1985;19:1557–1563
- Olofsson BO, Dahlen G, Nilsson TK. Evidence for increased levels of plasminogen activator inhibitor and tissue plasminogen activator in plasma of patients with angiographically verified coronary artery disease. Eur Heart J. 1989;10:77–82
- Marvi A, Stegnar M, Krebs M, Sentocnik JT, Geiger M, Binder BR. Impact of adipose tissue on plasma plasminogen activator inhibitor-1 in dieting obese women. Arterscler Thromb Vasc Biol. 1989;19:1582–1587
- Mitrio V, Pergola G, Vettor R, et al. Plasma plasminogen activator inhibitor-1 is associated with plasma leptin irrespective of body mass index, body fat mass, and insulin and metabolic parameters in premenopausal women. Metabolism. 1989;48:960–964
- Heimburger O, Lönnqvist F, Danielson A, Nordenstrom J, Stenvinkel P. Serum immunoreactive leptin concentration and its relation to the body fat content in chronic renal failure. JASN. 1997;8:1423–1430
- Daschner M, Tönshoff B, Blum WF, et al. Inappropriate elevation of serum leptin levels in children with chronic renal failure. JASN. 1998;9:1074–1079
- Sharma K, Considine RV, Michael B, et al. Plasma leptin is partly cleared by the kidney and is elevated in hemodialysis patients. Kidney Int. 1997;51:1980–1985
- Howard JK, Lord GM, Clutterbuck EJ, Ghatei MA, Pusey CD, Bloom SR. Plasma immunoreactive leptin concentration in end-stage renal disease. Clin Sci(Lond). 1997;93:119–126
- Tsujimoto Y, Shoji T, Tabata T, et al. Leptin in peritoneal dialysate from continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1999;34:832–838
- Kagan A, Haran N, Leschinsky L, Shuali N, Rapaport J. Leptin in CAPD patients: serum concentration and peritoneal loss. Nephrol Dial Transplant. 1999;14:400–405
- Landt M, Parvin A, Dagogo S, Bryant B, Coyne DW. Leptin elimination in hyperleptinemic peritoneal dialysis patients. Nephrol Dial Transplant. 1999;14:731–737
- Arkouche W, Juillard L, Delawari E, et al. Peritoneal clearance of leptin in continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1999; 34:839–844
- Sinha MK, Opentanova I, Ohannesian JP, et al. Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting. J Clin Invest. 1996;98:1277–1282
- Widjaja A, Keilstein JT, Horn R, Mühlen A, Klein V, Brabant D. Free serum leptin but not bound leptin concentrations are elevated in patients with end stage renal disease. Nephrol Dial Transplant. 2000;15:846–850
- Malyszko J, Wolczynski S, Malyszko J, Mysliwiec M. Leptin correlates with hemostatic parameters in CAPD patients. Nephron. 2002;92:721–724
- Irish AB. Plasminogen activator inhibitor-1 activity in chronic renal disease and dialysis. Metabolism. 1997;46:36–40
- Gris JC, Branger B, Vecina F. Increased cardiovascular risk factors and features of endothelial activation and dysfunction in dialyzed uremic patients. Kidney Int. 1994;46:807–813
- Widjaja A, Stratton IM, Horn R, Holman RR, Turner R, Brabant G. UKPDS 20: plasma leptin, obesity, and plasma insulin in type 2 diabetic subjects. J Clin Endocrinol Metab. 1997;82:654–657
- Segarra A, Chagon P, Martinez-Eyarre C, et al. Circulating levels of plasminogen activator inhibitor-1, tissue plasminogen activator and thrombomodulin hemodialysis patients: biochemical correlations and role as independent predictors of coronary stenosis. J Am Soc Nephrol. 2001;12:1255–1263
- Haaber AB, Eidemak I, Jensen T, Feldt-Rasmussen B, Strangaard S. Vascular endothelial cell function and cardiovascular risk factors in patients with chronic renal failure. J Am Soc Nephrol. 1995;5:1581–1584
- Ishii Y, Yano S, Maezawa A, Tsuchida A, Wakamatsu R, Naruse T. Evaluation of blood coagulation-fibrinolysis system in patients receiving chronic hemodialysis. Nephron. 1996;73:407–412
- Aunsholt NA, Alhbom G, Steffensen G, Glud T. Fibrinolytic capacity in hemodialysis patients treated with recombinant erythropoietin. Nephron. 1992;62:284–288
- Opatrny KJ, Vit L, Opatrna S, et al. The effect of erythropoietin on fibrinolysis in hemodialyzed patients. Cas Lek Cesk. 1995;134:136–138
- Barawski J, Mysliwiec M. Effects of recombinant erythropoietin therapy on circulating endothelial markers in hemodialysis. Clin Appl Thromb Hemost. 2003;9:349–352