Abstract
Hexachlorobutadiene (HCBD) is a potent nephrotoxin which nowadays contaminates human foods and water. On the other hands, it has been reported that rutin is a chemopreventive flavonoid which exerts some protective effects on the kidney. Therefore, in this work, the possible effect of rutin on HCBD-induced nephrotoxicity was investigated in female rats. The animals were divided into five groups. Groups 1 and 2 were treated with vehicle and HCBD (100 mg/kg, i.p.), respectively. Groups 3–5 were pretreated with rutin (100, 500 and 1000 mg/kg, i.p.) 1 h before HCBD injection. The level of serum urea and creatinine as well as urinary glucose and protein were measured. Total thiol content and lipid peroxidation level were also determined in the kidney homogenate. When compared to control group, a significant increase in the level of serum creatinine and urea (p < 0.001) as well as urine glucose and protein (p < 0.001) were observed after 24 h of HCBD administration. HCBD also caused a significant decrease in total thiol content (p < 0.001) and a significant increase in lipid peroxidation level (p < 0.001). Pretreatment with rutin could decrease serum creatinine (p < 0.001) and urea (p < 0.001) as well as urine protein (p < 0.001) concentrations when compared with HCBD treated rats. No significant modification on urine glucose was seen (p > 0.05). Rutin also reversed the HCBD-induced depletion in thiol content (p < 0.001) and elevation in lipid peroxidation (p < 0.001) in the kidney. The results of present study showed that rutin clearly attenuated HCBD-induced nephrotoxicity and has the potential to be considered as a nephroprotective agent.
Introduction
Hexachlorobutadiene (HCBD) is a halogenated aliphatic compound which extensively is used in industry for composition of elastomers, rubbers, heat-transfer liquids, transformers, hydraulic fluids, insecticide, herbicide and fungicide.Citation1,Citation2 It is dispersed in the environment and contaminates human foods and water with measurable quantities. Several studies have shown that HCBD induces deleterious effects in the body due to its toxicityCitation3 Increased levels of reactive oxygen species and lipid peroxidation products involved in the toxicity of HCBD on the body.Citation4,Citation5 It has been well documented that HCBD is a potent nephrotoxin and its bioactivation results in formation of toxic electrophilic metabolites which causes degeneration of renal tubular epithelial cells.Citation6–8
Rutin (3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside) is a flavonoid constituent which abundantly found in some foods and plant-based beverages such as buckwheat, onions, apples, berries and tea.Citation9 It has several pharmacological effects including cytoprotective, anticarcinogenic, hepatoprotective, anticonvulsant and cardioprotective activities.Citation10–13 Experimental studies have revealed some renoprotective effects of rutin. Korkmaz and Kolankaya demonstrated that rutin attenuates renal injury following ischemia/reperfusion (I/R) condition probably by inhibiting inducible nitric oxide synthase (iNOS).Citation14,Citation15 Khan et al. also suggested that rutin prevent oxidative renal damage in rat treated with potassium bromate by antioxidant activity.Citation16 Arjumand et al. found that pretreatment with rutin inhibits cisplatin-induced renal toxicity in rats. This effect was accompanied by marked decrease in the peroxidative, inflammatory and apoptotic pathways.Citation17 Also, beneficial effects of rutin on streptozotocin (STZ)-induced nephrotoxicity have been shown mediated by reducing reactive oxygen species (ROS) and lipid peroxidation levels.Citation18 Moreover, it was shown that the preventive effect of rutin on the development of STZ-induced diabetic nephropathy is closely related to inhibit oxidative stress and the expression of connective tissue signaling pathways.Citation19 Therefore, the present study was designed to investigate the possible protective effects of rutin on HCBD-induced nephrotoxicity in rats.
Materials and methods
Chemicals and reagents
HCBD and rutin were obtained from Fluka (St. Gallen, Switzerland) and Sigma (St. Louis, MO), respectively. Tris (hydroxymethyl) aminomethane (Trizma base), 2,2′-dinitro-5,5′-dithiodibenzoic aid (DTNB), 2-thiobarbituric acid, n-butanol, ethylenediaminetetraacetic acid disodium salt (Na2EDTA), ether and tetramethoxypropane were purchased from Merck (Darmstadt, Germany).
Animal groups
Adult female Wistar rats weighing 250–300 g were used in this study. The animals were housed in a pathogen-free facility on a 12 h light/dark schedule and with ad lib access to food and water. All animal procedures were approved by the university ethics committee and were in compliance with national laws and with National Institutes of Health guidelines for the use and care of laboratory animals. After acclimatization, animals were divided into five groups (n = 6). Group 1 was treated with saline as control. Animals in group 2 received a single dose of HCBD (100 mg/kg, i.p.).Citation20,Citation21 Groups 3–5 were pretreated with rutin (100, 500 and 1000 mg/kg, i.p., respectively) 1 h before HCBD injection.Citation14,Citation15,Citation17 The animals were sacrificed under ether anesthesia 24 h after HCBD injection.
Blood and urine biochemical assays
Blood samples were taken out by cardiac puncture for measuring the level of serum urea and creatinine. Also, 24 h urine samples were collected and used for measuring glucose and protein concentration. Urea concentration was determined colorimetrically by Autoanalyzer (Technicon RA-1000, England) using urea kit (Man Lab Company, Tehran, Iran). Glucose level was measured by glucose oxidase reagent (Ziest Chem Diagnostics, Iran). Concentration of creatinine and protein was determined by the Jaffe’s technique and turbidimetric method, respectively.Citation22,Citation23
Lipid peroxidation assay
The lipid peroxidation level in the kidney homogenate sample was estimated by measuring malondialdehyde (MDA) which is the end product of lipid peroxidation. The MDA combines with 2-thiobarbituric acid as a thiobarbituric acid reactive substance to give a red colored complex which has peak absorbance at 530 nm.Citation24 In our experiment, 3 ml phosphoric acid (1%) and 1 ml thiobarbituric acid (0.6%) was added to 0.5 ml of tissue homogenate and the mixture was heated for 45 min in a boiling water bath. After cooling, 4 ml of n-butanol was added to the mixture and centrifuged at 5000 rpm for 10 min. The organic layer was transferred to a fresh tube and its absorbance was measured at 532 nm.
Measurement of total thiol content
Total sulfhydryl groups were measured using DTNB reagent. This reagent reacts with the sulfhydryl groups to produce a yellow colored complex which has peak absorbance at 412 nm. In our assay, 1 mL Tris-EDTA buffer (pH 8.6) was added to 50 μL kidney homogenate and sample absorbance was read at 412 nm against Tris-EDTA buffer alone (A1). Then 20 μL DTNB was added to the mixture and after 15 min the sample absorbance was read again (A2). The optical density of DTNB reagent was also read as a blank (B). Total thiol concentration was calculated from the following equation.Citation25
Statistical analysis
Data were expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey--Kramer post-hoc test for multiple comparisons. The p values less than 0.05 were considered to be statistically significant.
Results
Effect of rutin on serum urea and creatinine
As shown in , a significant elevation of serum urea (112 ± 2.8 mg/dL, p < 0.001) was observed 24 h after administration of HCBD as compared to control group (33 ± 1.5 mg/dL). Pretreatment with rutin at all concentrations caused a significant (p < 0.001) decrease in HCBD-induced hyperuremia.
Figure 1. Effects of rutin on concentration of serum urea (A) and creatinine (B) in rats treated with hexachlorobutadiene (HCBD). Rutin was administrated intraperitoneally 1 h before HCBD injection (100 mg/kg, i.p.). Control rats were received saline as vehicle. Data are shown as mean ± SEM (n = 6). #p < 0.001 compared to control; *p < 0.001 as compared with HCBD group.
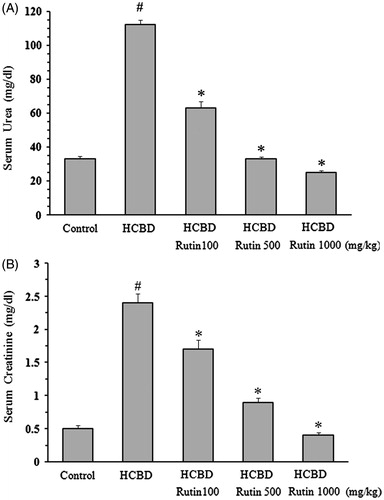
Injection of HCBD also resulted in sever increase in serum creatinine level (2.4 ± 0.14 mg/dL, p < 0.001) when compared with control animals (0.5 ± 0.05 mg/dL). Again, pretreatment with rutin significantly (p < 0.001) inhibited this effect of HCBD in a concentration-dependent manner ().
Effect of rutin on urine glucose and protein
A significant increase in the level of urine glucose was seen following HCBD injection compared to control rats (37 ± 3.3 vs. 8.2 ± 1.3 mg/dL, p < 0.001). Although rutin decreased concentration of urine glucose, but its effect was statistically insignificant ().
Figure 2. Effects of rutin on concentration of urine glucose (A) and protein (B) in rats treated with hexachlorobutadiene (HCBD). Rutin was administrated intraperitoneally 1 h before HCBD injection (100 mg/kg, i.p.). Control rats were received saline as vehicle. Data are shown as mean ± SEM (n = 6). #p < 0.001 compared to control; *p < 0.001 as compared with HCBD group.
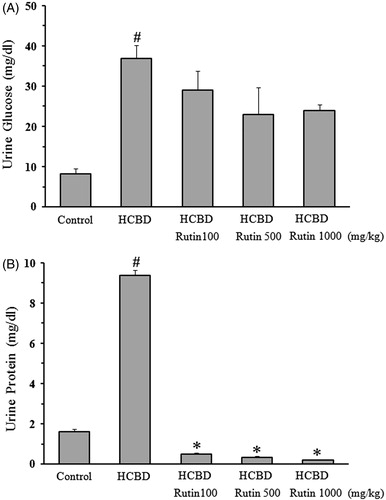
shows the effect of rutin on urine protein. In the groups received 100, 500 and 1000 mg/kg of rutin, the protein level was 0.5 ± 0.05, 0.3 ± 0.07 and 0.2 ± 0.04 mg/dL, respectively, which significantly lower than that of HCBD group (9.4 ± 0.26 mg/dL, p < 0.001).
Effect of rutin on lipid peroxidation
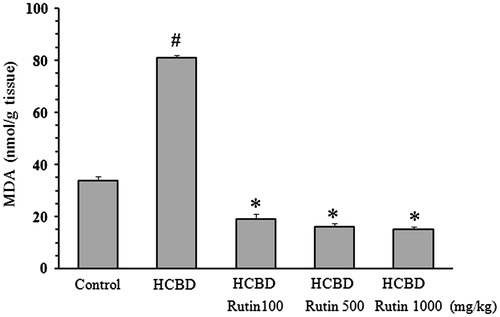
The level of MDA in kidney homogenate significantly increased in HCBD group (81 ± 1 nmol/g tissue, p < 0.001), when compared with that of control rats (34 ± 1.5 nmol/g tissue). The content of MDA was significantly decreased in the animals pretreated with 100 mg/kg (19 ± 2 nmol/g tissue, p < 0.001), 500 mg/kg (16 ± 1.5 nmol/g tissue, p < 0.001) or 1000 mg/kg (15 ± 1 nmol/g tissue, p < 0.001) of rutin ().
Figure 3. Effect of rutin on lipid peroxidation in the kidney homogenate of rats treated with hexachlorobutadiene (HCBD). Rutin was administrated intraperitoneally 1 h before HCBD injection (100 mg/kg, i.p.). Control rats were received saline as vehicle. The lipid peroxidation level was estimated by measuring the concentration of malondialdehyde (MDA). Data are shown as mean ± SEM (n = 6). #p < 0.001 compared to control; *p < 0.001 as compared with HCBD group.
Effect of rutin on total thiol content
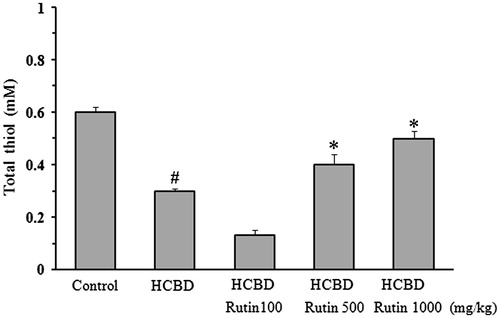
As shown in , a significant decrease (50%) in the content of kidney thiol was found in the animals received HCBD. Compared to control group (0.6 ± 0.02 mM), rutin at high concentrations could reverse the HCBD-induced depletion of thiol content (0.4 ± 0.04 and 0.5 ± 0.03 mM for 500 and 1000 mg/kg, respectively).
Figure 4. Effect of rutin on total thiol content in the kidney homogenate of rats treated with hexachlorobutadiene (HCBD). Rutin was administrated intraperitoneally 1 h before HCBD injection (100 mg/kg, i.p.). Control rats were received saline as vehicle. Data are shown as mean ± SEM (n = 6). #p < 0.001 compared to control; *p < 0.01 as compared with HCBD group.
Discussion
Our results, in agreement with previous studies, demonstrated that HCBD is a potent nephrotoxin compound.Citation6,Citation7,Citation26,Citation27 It increased urinary excretion of glucose and protein and elevated levels of serum urea and creatinine. The antioxidant status of kidney was also lowered in the HCBD treated animals. The increase in lipid peroxidation level and decrease in total sulfhydryl content suggest enhanced oxidative stress in the kidney following exposure to HCBD. This increase in lipid peroxidation has also been shown to be involved in the toxicity of HCBD in other tissues such as liver and brain.Citation4,Citation5 Sulfhydryl groups are highly-reactive constituents of protein and non-protein molecules and they participate in varying biochemical process including detoxification mechanisms.Citation28 The HCBD-induced sulfhydryl depletion in the kidney homogenate is also in accordance with the results of Kluwe et al. who showed single injection of HCBD decreased hepatic and renal sulfhydryl concentrations.Citation29
Previous studies have shown that renal toxicity of HCBD is due to its conjugation with glutathione to form glutathione S-conjugate by glutathione S-transferase. Subsequently, further processing by the enzymes of the mercapturic acid pathway leads to formation of toxic electrophilic intermediates which damage renal epithelial cells by a combination of covalent modification of macromolecules, thiol depletion and initiation of lipid peroxidation.Citation7,Citation30–32
Our present results demonstrated that rutin improved the HCBD-induced abnormalities in renal parameters (serum urea and creatinine and urinary protein), which are in agreement with previous reports. Rutin has been shown to improve renal dysfunction induced by numerous conditions such as high fructose or cholesterol diet, ischemia--reperfusion injury or chemotherapeutic agents like doxorubicin or cisplatin.Citation14,Citation33–35
In this work, we also showed that rutin can reverse the HCBD-induced depletion of thiol content and restore the increased level of lipid peroxidation. It has been reported that rutin acts as anti-lipoperoxidant and free radicals scavenger agent.Citation36,Citation37 A similar decrease in renal dysfunction and oxidative stress has been shown in rutin treated diabetic rats.Citation38 Same authors also reported that rutin, by improving the renal antioxidant status and reducing the plasma glucose levels, effectively prevent matrix remodelling in the kidney of streptozotocin-induced diabetic rats.Citation39,Citation40 It has been shown that rutin can prevent the development of experimental diabetic nephropathy by decreasing the oxidative stress and accumulation of extracellular matrix components in rats, in vitro or in vivo.Citation19,Citation41 Furthermore, it was reported that rutin attenuate renal ischemia--reperfusion injury as a result of reduction the oxidative stress markers and inhibition of inducible nitric oxide synthase (iNOS).Citation15 Also, our findings are in accordance with those reported by Arjumand et al. who demonstrated an inhibitory effect of rutin on the level of lipid peroxidation products in the kidney of cisplatin treated rats.Citation17
There are several studies in which the anti-inflammatory and anti-apoptotic properties of rutin has been demonstrated. It was noted that rutin at 50 and 100 mg/kg inhibited renal NOD-like receptor 3 (NLRP3) inflammasome activation, renal pro-inflammatory cytokines overproduction, inflammation and lipid accumulation in the kidney of fructose-fed rats.Citation42 Similarly, Arjumand et al. found that the beneficial effect of rutin pretreatment is mediated partially by its inhibitory effect on NFκB and TNF-α pathway mediated inflammation, caspase-3 mediated-tubular cell apoptosis, as well as by restoration of antioxidant enzymes activity and renal functional parameters.Citation17
Beside its antioxidant activities, protective effect of rutin against HCBD-induced nephrotoxicity can be achieved through inhibition of enzymes involved in the bioactivation of HCBD. Glutathione-S-transferase is of the main enzymes responsible for nephrotoxicity of HCBD and its urinary excretion is recommended as sensitive biomarker of HCBD-induced renal toxicity.Citation8 Interestingly, inhibitory effect of quercetin, the active metabolite of rutin, on glutathione-S-transferase activity was shown in both rat and human.Citation28,Citation29
In conclusion, the results of present study showed that rutin attenuates HCBD-induced nephrotoxicity. This is supported by improvements of renal functional tests and by decrease of renal oxidative stress. Therefore, it has the potential to be considered as a potent nephroprotective agent.
Declaration of interest
This work was supported by a grant from Research Council of Mashhad University of Medical Sciences, Mashhad, Iran.
References
- Anonymous. Chemical review, hexacholoro-1,3-butadiene. Danger Prop Indus Mater Rep. 1992;12:2–23
- Verschueren K. Handbook of Environmental Data on Organic Chemicals. 3rd ed. New York, NY: Van Nostrand Reinhold; 1996:1070–1072
- Green T, Lee R, Farrar D, Hill J. Assessing the health risks following environmental exposure to hexacholoro-1,3-butadiene. Toxicol Lett. 2003;138:63–73
- Almeida MG, Fanini F, Davino SC, Aznar AE, Koch OR, Barros SB. Pro- and anti-oxidant parameters in rat liver after short term exposure to hexachlorobenzene. Hum Exp Toxicol. 1997;16:257–261
- Song SB, Xu Y, Zhou BS. Effects of hexachlorobenzene on antioxidant status of liver and brain of common carp (Cyprinus carpio). Chemosphere. 2006;65:699–706
- Boroushaki MT, Mofidpour H, Sadeghnia HR. Protective effect of safranal against hexacholoro-1,3-butadiene-induced nephrotoxicity in rat. Iran J Med Sci. 2007;32:173–176
- Lock EA, Ishmael J. The acute toxic effects of hexachloro-1: 3-butadiene on the rat kidney. Arch Toxicol. 1979;43:47–57
- Swain A, Turton J, Scudamore C, et al. Nephrotoxicity of hexachloro-1:3-butadiene in the male Hanover Wistar rat; correlation of minimal histopathological changes with biomarkers of renal injury. J Appl Toxicol. 2012;32:417–428
- Manach C, Morand C, Demigné C, Texier O, Régérat F, Rémésy C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997;409:12–16
- Janbaz KH, Saeed SA, Gilani AH. Protective effect of rutin on paracetamol- and CCl4-induced hepatotoxicity in rodents. Fitoterapia. 2002;73:557–563
- Nassiri-Asl M, Shariati-Rad S, Zamansoltani F. Anticonvulsive effects of intracerebroventricular administration of rutin in rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:989–993
- Ramos AA, Lima CF, Pereira ML, Fernandes-Ferreira M, Pereira-Wilson C. Antigenotoxic effects of quercetin, rutin and ursolic acid on HepG2 cells, evaluation by the comet assay. Toxicol Lett. 2008;177:66–73
- Trumbeckaite S, Bernatoniene J, Majiene D, Jakstas V, Savickas A, Toleikis A. The effect of flavonoids on rat heart mitochondrial function. Biomed Pharmacother. 2006;60:245–248
- Korkmaz A, Kolankaya D. Protective effect of rutin on the ischemia/reperfusion induced damage in rat kidney. J Surg Res. 2010;164:309–315
- Korkmaz A, Kolankaya D. Inhibiting inducible nitric oxide synthase with rutin reduces renal ischemia/reperfusion injury. Can J Surg. 2013;56:6–14
- Khan RA, Khan MR, Sahreen S. Protective effects of rutin against potassium bromate induced nephrotoxicity in rats. BMC Complement Altern Med. 2012;12:204--215
- Arjumand W, Seth A. Sultana S. Rutin attenuates cisplatin induced renal inflammation and apoptosis by reducing NFκB, TNF-α and caspase-3 expression in wistar rats. Food Chem Toxicol. 2011;49:2013–2021
- Alsaif MA. Beneficial effects of rutin and vitamin C coadministration in a streptozotocin-induced diabetes rat model of kidney nephrotoxicity. Pak J Nutr. 2009;8:745–754
- Hao HH, Shao ZM, Tang DQ, et al. Preventive effects of rutin on the development of experimental diabetic nephropathy in rats. Life Sci. 2012;91:959–967
- Bouroshaki MT, Sadeghnia HR, Banihasan M, Yavari S. Protective effect of pomegranate seed oil on hexachlorobutadiene-induced nephrotoxicity in rat kidneys. Ren Fail. 2010;32:612–617
- Cristofori P, Zanetti E, Fregona D, Piaia A, Trevisan A. Renal proximal tubule segment-specific nephrotoxicity: an overview on biomarkers and histopathology. Toxicol Pathol. 2007;35:270–275
- Masson P, Ohlsson P, Bjorkhem I. Combined enzymatic-Jaffe’s method for determination of creatinine in serum. Clin Chem. 1981;27:18–21
- McElderry LA, Tarbit IF, Cassells-Smith AJ. Six methods for urinary protein compared. Clin Chem. 1982;28:356–360
- Fernandez J, Perez-Alvarez JA, Fernandez-lopez JA. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997;99:345–353
- Sadeghnia HR, Kamkar M, Assadpour E, Boroushaki MT, Ghorbani A. Protective effect of safranal, a constituent of Crocus sativus, on quinolinic acid-induced oxidative damage in rat hippocampus. Iran J Basic Med Sci. 2013;16:73–82
- Berndt WO, Mehendale HM. Effects of hexachlorobutadiene (HCBD) on renal function and renal organic ion transport in the rat. Toxicology. 1979;14:55–65
- Boroushaki MT, Mofid Pour H, Dolati K. Protective effects of Verapamil against hexachlorobutadiene nephrotoxicity in rat. Iran J Med Sci. 2004;29:101–104
- Meyer AJ, Hell R. Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth Res. 2005;86:435–457
- Kluwe WM, McNish R, Smithson K, Hook JB. Depletion by 1,2-dibromoethane, 1,2 dibromo-3-chloropropane, tris (2,3-dibromopropyl) phosphate, and hexachloro-1,3-butadiene of reduced non-protein sulfhydryl groups in target and non-target organs. Biochem Pharmacol. 1981;30:2265–2271
- Reichert D, Schutz S. Mercapturic acid formation is an activation and intermediary step in the metabolism of hexacholoro-1, 3-butadiene. Biochem Pharmacol. 1986;35:1271–1275
- Kim HS, Cha SH, Abraham DG, Cooper AJ, Endou H. Intranephron distribution of cysteine S-conjugate β-lyase activity and its implication for hexachloro-1,3-butadiene-induced nephrotoxicity in rats. Arch Toxicol. 1997;71:131–141
- Boroushaki MT. Development of resistance against hexachlorobutadiene in the proximal tubules of young male rat. Comp Biochem Physiol C Toxicol Pharmacol. 2003;136:367–375
- Hu QH, Wang C, Li JM, Zhang DM, Kong LD. Allopurinol, rutin, and quercetin attenuate hyperuricemia and renal dysfunction in rats induced by fructose intake: renal organic ion transporter involvement. Am J Physiol Renal Physiol. 2009;297:F1080–F1091
- Al-Rejaie SS, Abuohashish HM, Alkhamees OA, Aleisa AM, Alroujayee AS. Gender difference following high cholesterol diet induced renal injury and the protective role of rutin and ascorbic acid combination in Wistar albino rats. Lipids Health Dis. 2012;11:41–51
- Peng CC, Hsieh CL, Ker YB, Wang HY, Chen KC, Peng RY. Selected nutraceutic screening by therapeutic effects on doxorubicin-induced chronic kidney disease. Mol Nutr Food Res. 2012;56:1541–1558
- Nègre-Salvayre A, Affany A, Hariton C, Salvayre R. Additional antilipoperoxidant activities of alpha-tocopherol and ascorbic acid on membrane-like systems are potentiated by rutin. Pharmacology. 1991;42:262–272
- Nègre-Salvayre A, Mabile L, Delchambre J, Salvayre R. alpha-Tocopherol, ascorbic acid, and rutin inhibit synergistically the copper-promoted LDL oxidation and the cytotoxicity of oxidized LDL to cultured endothelial cells. Biol Trace Elem Res. 1995;47:81–91
- Kamalakkannan N, Stanely Mainzen Prince P. Rutin improves the antioxidant status in streptozotocin-induced diabetic rat tissues. Mol Cell Biochem. 2006;293:211–219
- Kamalakkannan N, Prince PS. Anti-hyperglycemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic Wistar rats. Basic Clin Pharmacol Toxicol. 2006;98:97–103
- Kamalakkannan N, Stanely Mainzen Prince P. The influence of rutin on the extracellular matrix in streptozotocin-induced diabetic rat kidney. J Pharm Pharmacol. 2006;58:1091–1098
- Tang DQ, Wei YQ, Gao YY, et al. Protective effects of rutin on rat glomerular mesangial cells cultured in high glucose conditions. Phytother Res. 2011;25:1640–1647
- Hu QH, Zhang X, Pan Y, Li YC, Kong LD. Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats. Biochem Pharmacol. 2012;84:113–125
- Van Zanden JJ, Hamman OB, van Iersel MLPS, et al. Inhibition of human glutathione S-transferase P1-1 by the flavonoid quercetin. Chemico-Biol Interaction. 2003;145:139–148
- Zhang K, Das NP. Inhibitory effects of plant polyphenols on rat liver glutathione S-transferases. Biochem Pharmacol. 1994;47:2063–2068
