Abstract
Aluminum phosphide is most common cause of poisoning in northern India. There is no specific antidote available and management of such cases is mainly supportive with high mortality. We present two cases of severe acute aluminium phosphide poisoning where continuous renal replacement therapy (CRRT) was started early along with other resuscitative measures and both the patients survived.
Introduction
We present two cases of acute aluminium phosphide (AP) poisoning who presented in shock after average 3 h of ingestion. Continuous renal replacement therapy (CRRT) was started early along with other resuscitative measures and we managed to survive both of these patients.
Case 1
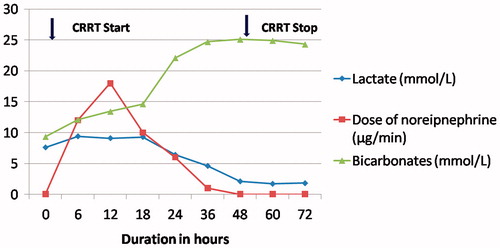
A 30-year-female presented 3 h after ingestion of AP half tablet (1.5 gm). Patient had three episodes of vomiting after consumption. On examination, patient was conscious, anxious with heart rate of 118/min, respiratory rate of 24/min and blood pressure of 96/60 mm Hg. Arterial blood gas showed metabolic acidosis with lactate of 7.4 (). Along with fluid resuscitation and vasopressors, gastric lavage was done with 150 ml coconut oil, 50 mEq sodium bicarbonate and 50 gm of charcoal. She was started on sodium bicarbonate infusion at 30 mEq/h and was given 4 gm of 50% magnesium sulfate to target serum magnesium 2–4 mmol/L. She was started on continuous veno-venous hemodiafiltration (CVVHDF) with femoral venous access and using Primasol® (Gambro) as dialysate and substitution fluid with a high flux filter. The blood flow rate was 150 mL/min and dialysate and replacement flow rate were1500 mL/h each. Initial echocardiography (ECHO) showed left ventricular ejection fraction (LVEF) of 50% which dropped to 35% after 24 h of admission. She was started on dobutamine infusion at 3 µg/kg/min. On day 3, vasopressors and CVVHDF were stopped and she was discharged on day 5 in stable state.
Case 2
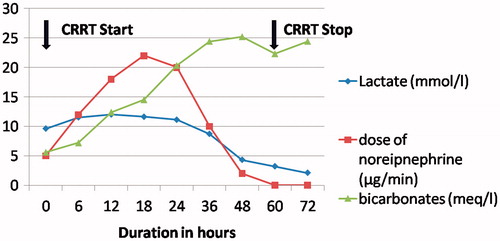
A 24-year-male was admitted 2.5 h after ingestion of 1 tab (3 gm) of AP with history of recurrent vomiting and pain in the epigastric region. On examination, he was conscious with heart rate of 136/min with frequent atrial ectopics on electrocardiogram (ECG). His blood pressure was 89/61 mm Hg and SpO2 was 94% on room air. Systemic examination was unremarkable. His initial arterial blood gas showed severe metabolic acidosis with lactate 9.6 mmol/L (). Fluid resuscitation was started and gastric lavage was done with 50 ml of coconut water and 50 mEq of sodium bicarbonate followed by continuous infusion at 25 mEq/h. He was started on CVVHDF using Primasol® (Gambro) as dialysate and substitution fluid 2 h after admission, in view of persistent severe metabolic acidosis (). The blood flow rate was 140 mL/min and dialysate and replacement flow rate were 1800 mL/hour each. Norepinephrine was added to target mean arterial pressure between 65 and 70 mm Hg. His ECHO showed global hypokinesia with LVEF 40%. He had episode of atrial fibrillation which was reverted with electrical cardioversion. His vasopressors requirement increased over 18 h with serial electrocardiogram (ECG) showing ST elevation in leads II, III, aVF and V4–V6. Review ECHO showed LVEF dropped to 15–20%. Intra-aortic balloon pump (IABP) with 1:2 augmentation was inserted through right femoral artery access and Swan Ganz catheter was inserted for cardiac output monitoring. He went into acute left ventricular failure for which non invasive ventilation was started. Thirty six hours after ingestion, his serum lactates and metabolic acidosis started improving. Vasopressors were tapered off on day 2 and IABP was removed on day 3. CVVHDF was stopped after 72 h. The patient was discharged on day 7.
Discussion
AP a potent grain fumigant and rodenticide, is most common cause of human poisoning in northern India.Citation1 It is available as dark brown or grayish tablets of 3 gm each. On ingestion, AP reacts with hydrochloric acid of stomach and liberates highly toxic phosphine gas. The exact mechanism of toxicity in human is still unclear. There are various mechanisms postulated that includes inhibition of cytochrome C oxidase, oxidative stress and extra-mitochondrial release of free oxygen radicals that causes ineffective adenosine triphosphate (ATP) production, lipid peroxidation and protein denaturation of the cell membrane in various organs.Citation2–4 The organs predominantly affected are heart, lungs, kidneys and gastrointestinal tract.Citation4 The most common clinical features include nausea, vomiting, abdominal pain.Citation3 The cardiac manifestations are common in severe poisoning which include profound hypotension, dry pericarditis, myocarditis, acute congestive heart failure and ECG abnormalities like dysrhythmias, ST-T wave changes and conduction defects.Citation3,Citation4 Other clinical features are respiratory distress, headache, dizziness, convulsion, encephalopathy and coma.Citation3 The rare clinical features include disseminated intravascular coagulation, hepatic necrosis and renal failure.Citation2,Citation4
The diagnosis is clinical with positive history of ingestion, typical clinical features, garlicky odor, arrhythmias and profound shock.Citation2–4 The mortality rate of AP poisoning is 31% even in tertiary care centers with fatal dose between 0.15 and 0.5 grams.Citation5 The most common cause of mortality is cardiogenic shock in these patients.Citation2,Citation4
The pathophysiology of cardiogenic shock is direct myocardial toxicity of phosphine gas and severe metabolic acidosis which further suppresses myocardial contractility thus perpetuating the vicious circle of refractory shock and death.Citation2,Citation4 The scores like SAPS II (Simplified Acute Physiology Score II) and APACHE II (Acute Physiology and Chronic Health Evaluation II) within the first 24 h can be used for assessment of severity and prediction of outcome.Citation6,Citation7 Our patients had APACHE II score of 15 and 13; SAPS II score of 30 and 34 respectively and both of them developed shock with requirement of vasopressors.
The management of AP poisoning is supportive as no antidote is available. The new therapeutic strategies tried in AP poisoning with varied success includes magnesium supplementation,Citation8 N-acetylcysteine,Citation9 hyperbaric oxygen,Citation10 IABP,Citation11 gastric lavage with mixture of coconut oil and bicarbonate solution,Citation12 almond oilCitation13 and digoxin.Citation14 However, either these strategies have not been tried in humansCitation9,Citation10,Citation13 or controlled human trials have failed to produce results consistently.Citation15 Then other therapies like enteral coconut oil, bicarbonate solution, potassium permanganate are useful early before absorption of phosphine gas, especially within 1–2 h of ingestion.Citation4,Citation12 The mainstay of the therapy in patients presenting latter or with shock is aggressive resuscitation and institution of supportive measures.Citation2–4 The poor prognostic markers of severe AP poisoning include profound hypotension, metabolic acidosis, ECG abnormalities, coma, severe hypoxia, gastrointestinal bleeding, and pericarditis.Citation4,Citation16 The correction of metabolic acidosis has shown to improve outcomes at least in one controlled trial.Citation17 It has been recommended that severe metabolic acidosis must be treated by administering sodium bicarbonate in patients with profound shock.Citation2,Citation4,Citation16
In our patients who presented we started resuscitation within first hour of their resuscitation and also we started CRRT within 2 h of their presentation after initial reports of high lactates were available. CRRT (CVVHDF) was done with bicarbonate based fluid for both substitute and dialysate and was continued till resolution of metabolic acidosis and normalization of serum lactates. Both the patients survived and we postulate that CRRT helped in these patients by correction of severe metabolic acidosis and hence stabilization of hemodynamics.
CRRT has an edge over intermittent hemodialysis in these patients can be used in case of hemodynamically unstable patients for prolonged duration where conventional hemodialysis is relatively contraindicated. Furthermore, CRRT helped to maintain metabolic milieu and resolution of shock state till AP got excreted. The studies have shown that CRRT which uses hemodiafiltration with convection as a principle scores over hemodialysis which is a diffusion based therapy.Citation18 We recommend further research on use of such extracorporeal therapies for these patients who have very high mortality with supportive management.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Singh D, Dewan I, Pandey AN, Tyagi S. Spectrum of unnatural fatalities in the Chandigarh zone of north-west India – a 25 year autopsy study from a tertiary care hospital. J Clin Forensic Med. 2003;10:145–152
- Bumbrah GS, Krishan K, Kanchan T, Sharma M, Sodhi GS. Phosphide poisoning: a review of literature. Forensic Sci Int. 2012;214:1–6
- Anand R., Binukumar BK, Gill KD. Aluminum phosphide poisoning: an unsolved riddle. J Appl Toxicol. 2011;31:499–505
- Mehrpour O, Jafarzadeh M, Abdollahi M. A systematic review of aluminium phosphide poisoning. Arh Hig Rada Toksikol. 2012;63:61–73
- Shadnia S, Sasanian G, Allami P, et al. A retrospective 7-years study of aluminum phosphide poisoning in Tehran: opportunities for prevention. Hum Exp Toxicol. 2009;28:209–213
- Mathai A, Bhanu MS. Acute aluminium phosphide poisoning: can we predict mortality? Indian J Anaesth. 2010;54:302–307
- Shadnia O, Mehrpour K, Shadnia S, Mehrpour O, Soltaninejad K. A simplified acute physiology score in the prediction of acute aluminum phosphide poisoning outcome. Indian J Med Sci. 2010;64:532–529
- Baeeri M, Shariatpanahi M, Baghaei A, et al. On the benefit of magnetic magnesium nanocarrier in cardiovascular toxicity of aluminum phosphide. Toxicol Ind Health. 2013;29:126–135
- Azad A, Lall SB, Mittra S. Effect of N-acetylcysteine and L-NAME on aluminium phosphide induced cardiovascular toxicity in rats. Acta Pharmacol Sin. 2001;22:298
- Saidi H, Shokraneh F, Ghafouri HB, Shojaie SJ. Effects of hyperbaric oxygenation on survival time of aluminum phosphide intoxicated rats. J Res Med Sci. 2011;16:1306–1312
- Siddaiah L, Adhyapak S, Jaydev S, et al. Intra-aortic balloon pump in toxic myocarditis due to aluminum phosphide poisoning. J Med Toxicol. 2009;5:80–83
- Shadnia S, Rahimi M, Pajoumand A, Rasouli MH, Abdollahi M. Successful treatment of acute aluminium phosphide poisoning: possible benefit of coconut oil. Hum Exp Toxicol. 2005;24:215–218
- Saidi H, Shojaie S. Effect of sweet almond oil on survival rate and plasma cholinesterase activity of aluminum phosphide-intoxicated rats. Hum Exp Toxicol. 2012;31:518–522
- Sanaei-Zadeh H, Farajidana H. Is there a role for digoxin in the management of acute aluminum phosphide poisoning? Med Hypotheses. 2011;76:765–766
- Chugh SN, Kamar P, Sharma A, Chugh K, Mittal A, Arora B. Magnesium status and parenteral magnesium sulphate therapy in acute aluminum phosphide intoxication. Magnes Res. 1994;7:289–294
- Wahab A, Zaheer MS, Wahab S, Khan RA. Acute aluminium phosphide poisoning: an update. Hong Kong J Emerg Med. 2008;15:152–155
- Jaiswal S, Verma RK, Tewari N. Aluminum phosphide poisoning: effect of correction of severe metabolic acidosis on patient outcome. Indian J Crit Care Med. 2009;13:21–24
- Prowle JR, Bellomo R. Continuous renal replacement therapy: recent advances and future research. Nat Rev Nephrol. 2010;6:521–529