Abstract
We aimed to investigate the performance of various creatinine based glomerular filtration rate estimation equations that were widely used in clinical practice in Turkey and calculate a correction coefficient to obtain a better estimate using the isotope dilution mass spectrometry (IDMS)-traceable Modification of the Diet in Renal Disease (MDRD) formula. This cross-sectional study included adult (>18 years) outpatients and in patients with chronic kidney disease as well as healthy volunteers. Iohexol clearance was measured and the precisions and bias of the various estimation equations were calculated. A correction coefficient for the IDMS-traceable MDRD was also calculated. A total of 229 (113 male/116 female; mean age 53.9 ± 14.4 years) subjects were examined. A median iohexol clearance of 39.21 mL/min/1.73 m2 (range: 6.01–168.47 mL/min/1.73 m2) was found. Bias and random error for the IDMS-traceable MDRD equation were 11.33 ± 8.97 mL/min/1.73 m2 and 14.21 mL/min/1.73 m2, respectively. MDRD formula seems to provide the best estimates. To obtain the best agreement with iohexol clearance, a correction factor of 0.804 must be introduced to IDMS-traceable MDRD equation for our study population.
Introduction
Chronic kidney disease (CKD) is a worldwide health problem. Various epidemiologic studies showed a prevalence of CKD of around 10% and a glomerular filtration rate (GFR) below 60 mL/min per 1.73 m2 of around 3%.Citation1 A recent study showed that the prevalence of CKD was as high as 15.7% in the adult population of Turkey.Citation2 Early detection and treatment of CKD may prevent or delay the development of chronic kidney failure and its various co-morbidities.Citation3,Citation4
CKD diagnosis is generally based on persistent albuminuria and/or decreased GFR. Assessment of GFR is indispensable for the evaluation of the presence and the severity of CKD.Citation5
GFR can be directly measured using renal clearance of inulin or plasma clearance of iohexol or iothalamate.Citation6,Citation7 However, these methods are costly, time-consuming, and require specific and advanced equipment not widely available and are therefore cumbersome, especially for community screening.Citation5 GFR is generally estimated using creatinine-based estimation equations. The time tested and widely used Cockroft-Gault and Modification of Diet in Renal Disease (MDRD) formulae are the two most commonly used estimation equations. Until recently, abbreviated MDRD formula has been championed by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI). However, the novel CKD-epi formula seems to out-perform the MDRD formula.Citation8
Creatinine-based formulae, however, also have limitations.Citation5 Variations in creatinine measurement greatly affect the predictive performance of the formulae and ethnicity affects the accuracy of the formula even after creatinine calibration.Citation9–11
We performed this study to evaluate the accuracy of widely used estimation equations and to devise a modification of the MDRD formula that can be used in population screening in Turkey.
Materials and methods
Study protocol
Eight subgroups were formed according to sex (male, female), age (<60 years, ≥60 years) and GFR (<40 mL/min, ≥40 mL/min). According to central limit theorem, a minimum of 30 patients were included in each subgroup.
Study participants were recruited from volunteer adults (≥18-years-old), participants evaluated in nephrology outpatient or inpatient clinics, potential kidney donors and hospital staff. A standard questionnaire was used to collect patient data; including sex, age, height, weight, and serum creatinine. Bedridden patients, pregnant patients, transplant recipients, patients with acute kidney failure, extremity amputation, malignancy, liver, thyroid or infectious diseases, or those with a history of allergic reaction to iodine contrast or of use radiocontrast agents in the previous 3 days were excluded from the study. Patients with previously diagnosed diabetes mellitus or those using oral antidiabetic drugs or insulin were accepted as diabetic. Hypertension was defined as consistently systolic blood pressure equal or greater than ≥140 mmHg, diastolic blood pressure of ≥90 mmHg, or the use of antihypertensive medications. Patients using antilipidemic drugs such as statins and fibrates were accepted as hyperlipidemic.
Study protocol was approved by the local medical ethics committee (approval no: 29787/2006). All participants gave written informed consent prior to their inclusion in the study. The study was performed in adherence to the 2000 Declaration of Helsinki.
Blood sample collection
An intravenous canula was placed in the peripheral vein of the upper arm. A blood sample was taken for the biochemical analysis, a solution of 10 mL iohexol (Omnipaque 300 mg/mL injection form; Amersham Healthcare, Cork, Ireland) was given, and the venous line was washed with saline. Blood samples were taken from the opposite arm.
Patients were divided into two groups according to the baseline estimated GFR calculated using the Cockroft-Gault formula. Time schedules used in this study were determined according to protocol described by Gaspari et al.Citation12 First, in patients with a GFR > 40 mL/min, blood samples were taken at 120, 150, 180, 210, and 240 min. Second, in patients with GFR ≤ 40 mL/min, blood samples were taken at 120, 180, 240, 300, 450, and 600 min.
Biochemical methods
GFR was determined by measuring the plasma clearance of iohexol which was measured using high-performance liquid chromatography (HPLC), according to the method of Soman et al.Citation13 Plasma samples were deproteinized by adding 100 µl plasma to 800 µl of 5% perchloric acid containing internal standard (Iohexol Related Compound C; Pharmacopeia, Rockville, MD). After 3 min of vortex and 5 min of sonication, the samples were centrifuged for 10 min in a microcentrifuge unit. The resulting supernatant was filtered from a 2 µm pore size pre-column filter and injected onto the analytical column. The separation was carried out on a reversed phase column, µ Bondapak C18 analytical column (150 × 3.9 mm, 10 µm particle size; Waters, Milford, MA). Iohexol was detected at 254 nm by a UV absorbance detector (Hewlett-Packard, Waldbroom, Germany).
HPLC grade acetonitrile (Merck KGaA, Darmstadt, Germany) was used as the mobile phase. The composition mobile phase was rapidly changed from an initial 4% acetonitrile to 14% acetonitrile in water from the fifth to the ninth minute. The concentration of the mobile phase was changed back to the initial 4% acetonitrile for 3 min. The flow rate was changed during the assay run from 0.8 mL/min for the initial 5 min to 1.2 mL/min for the intermediate 4 min, and back to 0.8 mL/min for 3 min.
Internal standard (1 mg/mL) and stock solution (10 mg/mL) of iohexol were prepared in acetonitrile. Calibrators and quality control samples were prepared from stock solution of iohexol. A total of five concentrations of iohexol of 10, 25, 50, 250, and 750 µg/mL in drug-free plasma were used as calibrators. The internal standard stock solution was diluted in 5% perchloric acid to produce a working strength (10 µg/mL) internal standard solution. All calculations were performed using peak area ratios of the larger iohexol peak to the internal standard peak (peak area ratio). The iohexol concentration was expressed as µg/mL. Iohexol clearance was calculated according to the methods described by Gaspari et al.Citation6
Albumin was measured with the colorimetric method using an Abbott C8000 automatic analyzer (Abbott Laboratories, Abbott Park, IL). Results were expressed as g/dL. Serum urea levels were measured using the urea enzymatic assay with the same autoanalyzer. Results were expressed as mg/dL.
Creatinine in serum was measured using the creatinine enzymatic assay (Jaffe rate method) in the Abbott C8000 analyzer. We used National Institute of Standards and Technology Standard Reference Material 967 (Gaithersburg, MD) to transform measured creatinine values to isotope dilution mass spectrometry (IDMS) traceable creatinine. Results were expressed as mg/dL.
Analytical and statistical methods
GFR measured by plasma iohexol clearance was accepted as the “gold standard”. GFR was calculated using the following estimation equations: Cockroft-Gault, MDRD 1 (six variables equation), MDRD 2 (four variables –abbreviated- equation), MDRD optimized for IDMS-traceable creatinine (IDMS-traceable MDRD), Jelliffe 1, Jelliffe 2, Mawer, Bjornsson, Gates, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI). GFR prediction equations based on serum creatinine level (Scr, mg/dL) are shown in .Citation14 It should be noted that some of these formulae () were devised to estimate creatinine clearance.
Table 1. GFR prediction equations based on serum creatinine level.
There is an inverse logarithmic correlation between creatinine and GFR. Therefore, we constructed different estimation equations using ln GFR and ln transformed values of the predictive variables based on regression analysis. MDRD formulae that use IDMS traceable creatinine are the current standard for estimation equations.Citation15 We used regression analysis to calculate a correction coefficient to obtain the best fit between the IDMS-traceable MDRD formula and the gold standard (iohexol clearance). We calculated the mean of iohexol clearance and various estimated GFR values for different GFR subgroups: <30 mL/min, 30–60 mL/min, >60 mL/min.
The difference between the estimated GFR by different creatinine-based equations and the iohexol clearance was calculated. Bias (systemic error) was calculated as the mean of these differences for each equation.
The agreement between GFR measured by iohexol and estimated GFR was evaluated using the Bland–Altman method.Citation16 According to this method, the graph showing the difference between two measurements versus the mean of the two measurements was drawn.
Precision was expressed by two different means; random error calculated as the mean of the absolute differences between the estimated GFR and iohexol measured GFR, and lower and upper limits of the Bland–Altman analysis. 95% agreement limits were expressed as ±1.96 SD of the mean differences.
Classification performance was determined according to NKF-KDOQI CKD groups.Citation17 In this manner, correct allocation of patients to each group for a specific equation was calculated.
Data was expressed as mean ± standard deviation. Statistical analyses were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL).
Results
Blood samples were collected from a total of 240 subjects for the measurement of iohexol clearance between March 2007 and November 2009. Eleven patients were excluded from the study because of technical reasons related to the laboratory. Pre-existing diabetes mellitus was present in 41 subjects (22 males, 19 females), hypertension in 147 subjects (73 males, 74 females), and hyperlipidemia in 42 subjects (25 males, 17 females). One hundred and three subjects (71 males, 32 females) were smokers.
The causes of CKD were hypertensive nephrosclerosis in 55 patients, chronic glomerulonephritis in 45, diabetes mellitus in 41, postrenal causes in 11, chronic interstitial nephritis in 7, AA-amyloidosis in 7, autosomal dominant polycystic kidney disease in 6, renal artery stenosis in 4, and was unknown in 18. Thirty-five subjects were healthy.
Clinical and laboratory characteristics of study subjects are shown in . Study participants were on average middle-aged (median: 54.5; range: 17–80). Serum creatinine values were between 0.57 and 8.25 mg/dL (median: 1.67 mg/dL). Baseline estimated GFR was ≥40 mL/min in 128 and <40 mL/min in 101 subjects.
Table 2. Clinical and laboratory characteristics of study subjects.
We devised the following formula to convert measured creatinine values to IDMS-traceable creatinine:
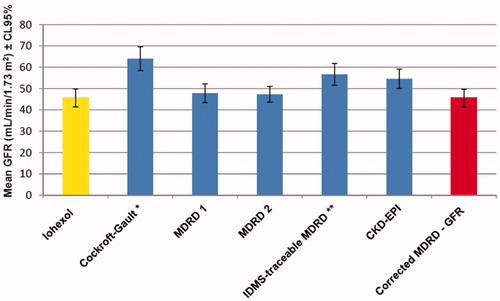
Mean iohexol clearance and results of various estimation equations are shown in . The median of GFR measured with iohexol clearance was 39.21 mL/min/1.73 m2 (range: 6.01 to 168.47 mL/min/1.73 m2). We found that the Cockroft-Gault, Mawer, Bjornsson and Gates formulae overestimated the mean GFR by more than 10 mL/min. The MDRD 2 equation, that uses the standardized creatinine, overestimated the GFR by 11 mL/min/1.73 m2. It should be noted that we did not find any formula that underestimated the GFR.
Table 3. Mean measured (iohexol) and estimated glomerular filtration rate values.
Bias, random error, precision lower and upper agreement limits and estimation equation accuracy are shown in . The largest and smallest bias and random errors were recorded with the Mawer and MDRD 2 formulae, respectively. The best precision and accuracy was also obtained with the MDRD 2 formula.
Table 4. The bias, random error, precision lower and upper agreement limits and accuracies of the estimation equations.
Confidence limits of the measured iohexol and estimated GFRs are shown in . MDRD 1 and MDRD 2 formulae were better than the others.
Figure 1. Iohexol measured and estimated glomerular filtration rate and confidence limits for various formulae (n = 229). Notes: GFR: Glomerular filtration rate, MDRD 1: Modification of Diet in Renal Disease 1 (for six variables), MDRD 2: Modification of Diet in Renal Disease 2 (for four variables), IDMS: Isotope dilution mass spectrometry, CKD - EPI: Chronic Kidney Disease Epidemiology Collaboration. *GFR was expressed as mL/min. **Isotope dilution mass spectrometry traceable creatinine was used.
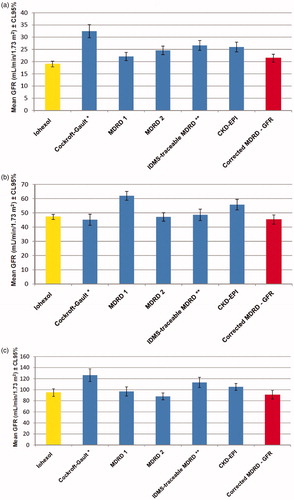
Estimated GFRs according to the different estimations equations tabulated by iohexol measured GFR values (<30 mL/min/1.73 m2, 30–60 mL/min/1.73 m2, >60 mL/min/1.73 m2) are shown in . The MDRD 2 formula performed better in patients with GFRs of 30–90 mL/min/1.73 m2 and the MDRD 1 formula in patients with GFR <30 mL/min/1.73 m2.
Figure 2. (a) The estimated glomerular filtration rates according to different estimations equations tabulated by iohexol measured glomerular filtration rate values (GFR < 30 mL/min/1.73 m2, n = 97). Notes: GFR: Glomerular filtration rate, MDRD 1: Modification of Diet in Renal Disease 1 (for six variables), MDRD 2: Modification of Diet in Renal Disease 2 (for four variables), IDMS: Isotope dilution mass spectrometry, CKD - EPI: Chronic Kidney Disease Epidemiology Collaboration. *GFR was expressed as mL/min. **Isotope dilution mass spectrometry traceable creatinine was used. (b) The estimated glomerular filtration rates according to different estimations equations tabulated by iohexol measured glomerular filtration rate values (GFR 30 – 60 mL/min/1.73 m2, n = 79). Notes: GFR: Glomerular filtration rate, MDRD 1: Modification of Diet in Renal Disease 1 (for six variables), MDRD 2: Modification of Diet in Renal Disease 2 (for four variables), IDMS: Isotope dilution mass spectrometry, CKD - EPI: Chronic Kidney Disease Epidemiology Collaboration. *GFR was expressed as mL/min. **Isotope dilution mass spectrometry traceable creatinine was used. (c) The estimated glomerular filtration rates according to different estimations equations tabulated by iohexol measured glomerular filtration rate values (GFR > 60 mL/min/1.73 m2, n = 53). Notes: GFR: Glomerular filtration rate, MDRD 1: Modification of Diet in Renal Disease 1 (for six variables), MDRD 2: Modification of Diet in Renal Disease 2 (for four variables), IDMS: Isotope dilution mass spectrometry, CKD - EPI: Chronic Kidney Disease Epidemiology Collaboration. *GFR was expressed as mL/min. **Isotope dilution mass spectrometry traceable creatinine was used.
The IDMS-traceable MDRD equation was multiplied by a correction coefficient of 0.804 to obtain the best fit with the iohexol measured GFR and the IDMS-traceable MDRD equation. Hence, the following formula was constructed:
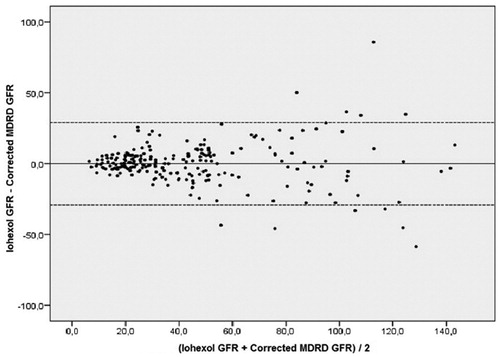
The Bland–Altman analysis showing the agreement between iohexol-measured GFR and corrected MDRD is shown in . There was a good agreement between the two methods, especially in patients with GFR < 60 mL/min/1.73 m2.
Figure 3. Bland–Altman analysis showing the agreement between iohexol measured glomerular filtration rate and modified MDRD formula (multiplied with the correction coefficient). Notes: GFR: Glomerular filtration rate, MDRD: Modification of Diet in Renal Disease.
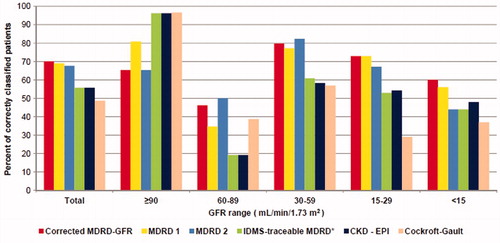
Classification performance of various estimation equations are shown in . The best classification performance was obtained with the corrected formula. Moreover, that formula showed the best classification performance in patients with GFR < 30 mL/min/1.73 m2.
Figure 4. Classification performance of various estimation equations. Notes: GFR: Glomerular filtration rate, MDRD 1: Modification of Diet in Renal Disease 1 (for six variables), MDRD 2: Modification of Diet in Renal Disease 2 (for four variables), IDMS: Isotope dilution mass spectrometry, CKD – EPI: Chronic Kidney Disease Epidemiology Collaboration. *Isotope dilution mass spectrometry traceable creatinine was used.
Discussion
In this study, we evaluated the performance of various creatinine based GFR estimation equations that were widely used in clinical practice in Turkey.
The selection of a reference GFR measurement method is critical for the evaluation of GFR estimation equations. We used the iohexol clearance as the gold standard for the determination of GFR. Iohexol is a contrast agent and its clearance has been well-correlated with standard GFR markers (such as inulin).Citation6,Citation14,Citation18 It is relatively inexpensive, non-radioactive, and safe to use in special patient populations, including those with severe renal insufficiency.Citation6,Citation14,Citation19,Citation20
The performance of an estimation equation can be expressed by two measures; bias (systemic; non-random deviations) and precision (spread of the series of the observations; random deviations). The performance of estimation equations depends on patient-related factors (age, gender, muscle mass, body composition and mass, nutrition, physical exercise, innate kidney function and underlying diseases), creatinine measurement method and specific GFR level.Citation14,Citation21 The effects of each of these factors on GFR are not mutually exclusive. For example, muscle mass is related to gender, age, nutritional status and physical exercise. The combined effect of patient-related factors can explain the well-documented ethnical differences on the performance of the creatinine-based estimated equations. The original MDRD study equation accurately estimated GFR in Caucasians but an additional corrective factor had to be used in black individuals.Citation22 Imai et al. found a significant discrepancy between the measured inulin clearance and estimated GFR by the MDRD or Cockroft-Gault equations in the Japanese population. The abbreviated MDRD study equation modified with the Japanese coefficient (0.881xMDRD) yielded lower mean difference and higher accuracy for GFR estimation.Citation23 Lee et al. reported that the Korean coefficients for the 4 and 6 variable MDRD and IDMS-MDRD study equations based on the serum creatinine recalibrated to CX3 analyzer and to the IDMS were 0.73989/0.74254 and 0.99096/0.9554, respectively.Citation24
Bias can also be related to differences in creatinine calibration. Recently, it has been suggested that all laboratories use creatinine methods calibrated to IDMS-traceable creatinine. In the US and other countries, nearly all methods have a calibration traceable to an IDMS reference measurement procedure.Citation25 In line with this suggestion, we also calibrated our creatinine measurements to IDMS-traceable creatinine and calculated a correction coefficient for the IDMS-traceable MDRD equation.
The performance of various estimation equations differs in several stages of CKD. According to Imai et al., even after modification, the abbreviated MDRD equation is still not optimal for estimation within the Japanese population because it underestimates the GFR above 60 mL/min/1.73 m2.Citation23 Bostom et al. compared standard iohexol GFR values with results of eight prediction equations in 109 patients with CKD and serum creatinine levels of <1.5 mg/dL. The most accurate results were obtained with the Cockroft-Gault and Bjornsson equations. The most precise formulae were the MDRD equations, although they were highly biased.Citation14 We tested our modified equation over the entire GFR range. We found that corrected MDRD formula overestimates in populations with GFR under 30 mL/min/1.73 m2 and underestimates in those over 30 mL/min/1.73 m2.
There were two main limitations to our study. First, the sample size was relatively small. Second, we used the study data to optimize the correction coefficient. However, we did not have a separate sample to test the validity of the coefficient.
We conclude that a multiplication of the IDMS-traceable MDRD equation by a coefficient of 0.804 produces a better classification performance over various GFR values in our population. However, another study with a validation sample is necessary to validate the wide use of the corrected formula.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This study was funded by the Turkish Society of Nephrology (project number: 18-3/2006).
Acknowledgements
The manuscript has been seen and approved by all authors and it is not under consideration for publication elsewhere in a similar form, in any language.
References
- Bello A, Kawar B, El Kossi M, El Nahas M. Epidemiology and pathophysiology of chronic kidney disease. In: Floege J, Johnson RJ, Feehally J, eds. Comprehensive Clinical Nephrology. St. Louis, Missouri: Elsevier Saunders; 2010:907–918
- Suleymanlar G, Utas C, Arinsoy T, et al. A population-based survey of Chronic REnal Disease In Turkey - the CREDIT study. Nephrol Dial Transplant. 2011; 26(6):1862–1871
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004; 351(13):1296–1305
- Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004; 351(13):1285–1295
- Agarwal R. Estimating GFR from serum creatinine concentration: pitfalls of GFR-estimating equations. Am J Kidney Dis. 2005; 45(3):610–613
- Gaspari F, Perico N, Ruggenenti P, et al. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol. 1995;6(2):257–263
- Gaspari F, Perico N, Remuzzi G. Measurement of glomerular filtration rate. Kidney Int. 1997;63:S151–154
- Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612
- Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39(5):920–929
- Hallan S, Asberg A, Lindberg M, Johnsen H. Validation of the Modification of Diet in Renal Disease formula for estimating GFR with special emphasis on calibration of the serum creatinine assay. Am J Kidney Dis. 2004;44(1):84–93
- Zuo L, Ma YC, Zhou YH, Wang M, Xu GB, Wang HY. Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis. 2005;45(3):463–472
- Gaspari F, Perico N, Matalone M, et al. Precision of plasma clearance of iohexol for estimation of GFR in patients with renal disease. J Am Soc Nephrol. 1998;9(2):310–313
- Soman RS, Zahir H, Akhlaghi F. Development and validation of an HPLC-UV method for determination of iohexol in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;816(1–2):339–343
- Bostom AG, Kronenberg F, Ritz E. Predictive performance of renal function equations for patients with chronic kidney disease and normal serum creatinine levels. J Am Soc Nephrol. 2002;13:2140–2144
- Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310
- Levey AS, Coresh J, Balk E, et al. National kidney foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147
- Brandstrom E, Grzegorczyk A, Jacobsson L, Friberg P, Lindahl A, Aurell M. GFR measurement with iohexol and 51Cr-EDTA: A comparison of the two favoured GFR markers in Europe. Nephrol Dial Transplant. 1998;13:1176–1182
- Olofsson P, Krutzen E, Nilsson-Ehle P. Iohexol clearance for assessment of glomerular filtration rate in diabetic pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64(1):63–67
- Sterner G, Frennby B, Hultberg B, Almen T. Iohexol clearance for GFR-determination in renal failure: single or multiple plasma sampling? Nephrol Dial Transplant. 1996;11(3):521–525
- Lee RC, Wang Z, Heo M, Ross R, Janssen I, Heymsfield SB. Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr. 2000; 72(3):796–803
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470
- Imai E, Horio M, Nitta K, et al. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2007;11(1):41–50
- Lee CS, Cha RH, Lim YH, et al. Ethnic coefficients for glomerular filtration rate estimation by the Modification of Diet in Renal Disease study equations in the Korean population. J Korean Med Sci. 2010;25(11):1616–1625
- Miller WG. Estimating glomerular filtration rate. Clin Chem Lab Med. 2009;47(9):1017–1019
