Abstract
Background: Transforming growth factor-β1 (TGF-β1) is a polypeptide member of the transforming growth factor β superfamily of cytokines and performs many cellular functions. Its overexpression may lead to renal fibrosis. Aim: This study planed to investigate the effects of TGF-β1 on the cell cycle and phenotype of mesangial cells. Methods: Rat mesangial cells were cultured together with different concentrations (0, 1, 2, 5, and 10 ng/mL) of TGF-β1 for specified times from 0 min to 72 h. 0 ng/mL TGF-β1 and 0 min served as controls. Cell cycles were assessed by flow cytometry and α-smooth muscle actin expression (α-SMA) protein expression by western blot analysis. All data were presented as Mean ± SD. Statistical analysis was performed by using one-way analysis of variance and correlation analysis. Results were considered significant at p < 0.05. Results: After 15 min of co-culture with different concentrations of TGF-β1, the percentage of mesangial cells in G0/G1 phase was significantly elevated compared to the control (p < 0.05). 12 h co-culture induced cell hyperplasia, 24 h co-culture obvious up-regulation of α-SMA (p < 0.01) and one or two cells’ myofibroblast phenotype transition, and 36 h co-culture several cells’ phenotype transition. Correlation analysis prompted that the TGF-β1-induced premature aging was time-dependent (p < 0.01). Conclusion: TGF-β1 may induce mesangial cells’ premature senescence and myofibroblast-like phenotype transformation time-dependently, which may contribute to the development of early stage of glomerulosclerosis.
Introduction
Mesangial cells are specialized smooth muscle cells around tiny blood vessels, or capillaries, in the kidney. They account for 30% ∼ 40% of intrinsic glomerular cell totals and help regulate the filtration process of blood while providing support for the glomerular structure.Citation1 They are also involved in the renal response to injury and disease. Under the pathological state, premature senescence and myofibroblast phenotypic changes may occur in the mesangial cells, contributing to the development and deterioration of glomerulosclerosis.Citation2 Transforming growth factor-β1 (TGF-β1) is a polypeptide member of the transforming growth factor β superfamily of cytokines and performs many cellular functions, such as the control of cell growth, cell proliferation, cell differentiation and apoptosis.Citation3 Many studies demonstrate that TGF-β1 is an important regulatory factor involved in the inflammatory damage and in the regulation of phenotype transdifferentiation of glomerular and tubular cells, and that the overexpression of TGF-β1 may lead to renal fibrosis.Citation4–Citation6 It has been proved that the surface of mesangial cells has the distribution of receptors of TGF-β1.Citation7,Citation8 Yet the effects of TGF-β1 on premature senescence and phenotype transformation of mesangial cells are still unclear. This study was planned to investigate this question via observing the changes of cell cycle and α-smooth muscle actin (α-SMA) protein expression of mesangial cells under the stimulation of TGF-β1.
Materials and methods
Cell line and cell culture
Rat mesangial cell line HBZY-1 used in this study was obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The mesangial cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Grand Island, NY) supplemented with 10% fetal calf serum (Gibco BRL, Grand Island, NY), 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mmol/L L-glutamine in humidified 5% CO2 incubator at 37 °C. Medium was changed every two or three days. 1 × 106 cells in the log phase were washed twice with PBS, seeded in a 100-mm tissue culture dish, and incubated with 3 ml of serum-free DMEM in humidified 5% CO2 incubator at 37 °C for 24 h. Subsequently, the 3 ml of serum-free DMEM in culture dish was replaced by 3 ml of media containing 0, 1, 2, 5, and 10 ng/mL human TGF-β1 (R&D Systems) respectively prepared in advance, and then the co-culture was performed in humidified 5% CO2 incubator at 37 °C within specified times, 0 min, 15 min, 30 min, 60 min, 12 h, 24 h, 36 h, 48 h and 72 h. The medium containing 0 ng/mL human TGF-β1 and the o min co-culture with TGF-β1 served as control.
Morphological evaluation of cells
After the co-culture under various conditions, the cells were examined under a phase contrast microscope (Japan Nikon) for alterations in size, shape, and integrity of cell membrane, cytoplasm and nucleus.
Cell cycle analysis
After the co-culture under various conditions, the cells were digested with 0.25% trypsin. The cell suspension was collected, centrifuged (1500 rpm, 5 min) and washed with PBS, and then the supernatant was discarded. The cells were fixed in 70% precooling ethanol (1-ml) for 1 ∼ 2 h at 4 °C. After fixation, the cells were re-suspended with PBS, treated with RNase (50 μg/mL), and re-suspended in PBS containing propidium iodide (0.05 mg/mL in 3.8 mol/L natrium citrate; Labest Biotechnology Co., Ltd., Beijing, China) at room temperature for 30 min. Then, the stained cells were analyzed using flow cytometry (FACS Caliber, Becton-Dickinson, San Jose, CA) according to the manufacturer's instructions and the data stored as list-mode files. DNA cell cycle histograms were analyzed and modeled using ModFit and WinList software (Verity Software House, Topsham, ME). Twenty thousand cells were analyzed in triplicate for each sample.
Detection of α-SMA expression
This study evaluated the phenotypic changes of mesangial cells via detecting the α-SMA protein expression, because α-SMA is the specific marker of myofibroblast phenotypic transformation of mesangial cells under pathological condition.Citation9 Western blot analysis was employed in this detection. After co-culture, the cells were lysed in RIPA lysis buffer (50 mM HEPES/NaOH, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 1% Triton X-100, and 200 μmol/L DTT) supplemented with protease inhibitor (1 mmol/L PMSF) for 30 min. Then the total protein was extracted and its concentration measured using BCA Protein Assay Kit (Thermo). The protein extracts were added to 5 × SDS sample buffer and boiled for 10 min. After that, 30 μg protein samples were put in the 10–15% polyacrylamide gel (SDS-PAGE, Amerisco) for electrophoresis (120 V, 70 min), and then were transferred to a PVDF (Immobilon-P™, Millipore) membrane (200 mA, 50 min, 4°C) and blocked with PBS containing 5% skim milk at room temperature for 2 h. Subsequently, the samples were added to the primary antibody (α-SMA: 1:1000, β-actin: 1:300) and kept overnight at 4 °C. After incubation with the horseradish peroxidase-labeled secondary antibody (1:25,000, Jackson ImmunoResearch) for 2 h, the samples were color-developed using ECL reagent (Pierce) and imaged. The films were scanned by using Adobe Photoshop software and analyzed using ImageJ.
Statistics
Data were presented as means ± SD. One way analysis of variance (ANOVA) and correlation analysis were used to do statistical analysis, p < 0.05 were considered statistically significant.
Results
Alteration of cell cycle
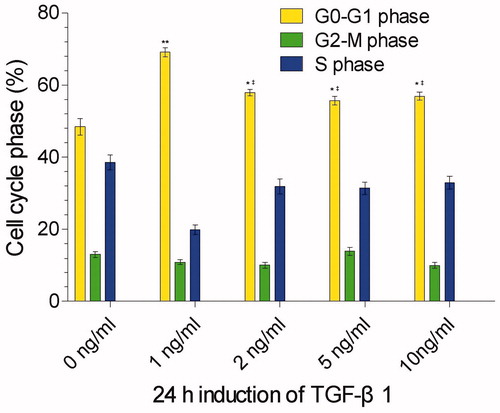
To know the effects of different concentrations of TGF-β1 on the cell cycle of mesangial cells, we co-cultured the mesangial cells with 0, 1, 2, 5, and 10 ng/mL TGF-β1 (0 ng/mL as control). 24 h after the co-culture, the percentage of mesangial cells in G0/G1 phase was increased significantly compared to the control (p < 0.01 or p < 0.05), indicating that TGF-β1 induced the cell cycle arrest in G0/G1 phase. Among the four concentrations of TGF-β1, 1 ng/mL TGF-β1 had the strongest effect to arrest the cell cycle, presenting with the highest percentage of mesangial cells in G0/G1 phase (69.15%). With the increase of TGF-β1 concentration (2, 5, 10 ng/mL), the percentage of mesangial cells in G0/G1 phase was decreased significantly when compared to the 1 ng/mL TGF-β1 concentration (p < 0.05) and reached the bottom at the concentration of 5 ng/mL TGF-β1, but there was no statistical difference between 2, 5, and 10 ng/mL TGF-β1 (p > 0.05) (). Correlation analysis revealed that no correlation existed in between the percentage of mesangial cells in G0/G1 phase and the concentrations of TGF-β1 (p > 0.05). This may suggest that the premature senescence of mesangial cells induced by TGF-β1 is not dose-dependent.
Figure 1. Cell cycle phases of mesangial cells 24 h after co-culture with 0, 1, 2, 5, and 10 ng/ml TGF-β1 (0 ng/ml serving as control). **stands for p < 0.01 and *p < 0.05 when compared to the control; ‡stands for p < 0.05 when compared to the 1 ng/ml TGF-β1.
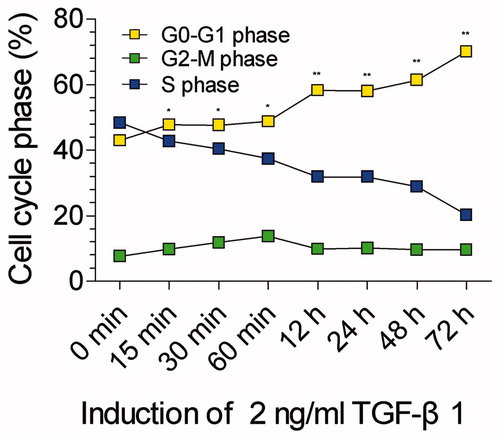
To know whether the premature senescence of mesangial cells induced by TGF-β1 is time-dependent or not, we adopted the fixed concentration of TGF-β1 (2 ng/mL) in media but different time points to detect the cell cycle, including 0 minute (as control), 15 min, 30 min, 60 min, 12 h, 24 h, 48 h, and 72 h. We found that 15 min after co-culture the percentage of mesangial cells in G0/G1 phase was elevated significantly compared to the control (p < 0.05) and it continued to elevate with the increase of time. Meanwhile the percentage of mesangial cells in S phase was decreased accordingly with the variation of time (). Correlation analysis revealed that the percentage of mesangial cells in G0/G1 phase was positively correlated with the culture time (p < 0.01). Evidently the premature senescence of mesangial cells induced by TGF-β1 is time-dependent.
Figure 2. Cell cycle phases of mesangial cells after co-culture with 2 ng/ml TGF-β1 in 0 min (as control), 15 min, 30 min, 60 min, 12 h, 24 h, 48 h, and 72 h. The percentage mesangial cells in G0/G1 phase is continuously increased since 15 min. **stands for p < 0.01 and *p < 0.05 when compared to the control.
Expression of α-SMA
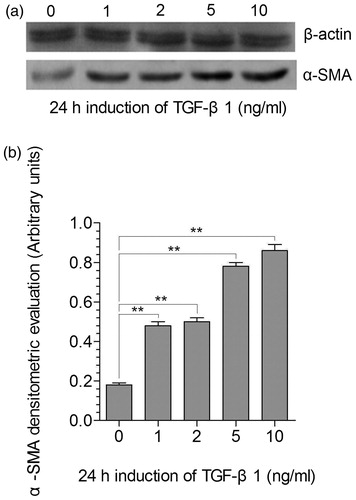
We detected the α-SMA expression in mesangial cells induced by 0, 1, 2, 5, and 10 ng/mL of TGF-β1 (0 ng/mL as control) (). Compared to the control, the α-SMA expression in mesangial cells was up-regulated under the induction of 1, 2, 5, and 10 ng/mL of TGF-β1 (p < 0.01) (). The up-regulation of α-SMA expression induced by 5 and 10 ng/mL of TGF-β1 was statistically higher than that by 1 and 2 ng/mL of TGF-β1 (p < 0.01). Correlation analysis prompted that the α-SMA expression was positively correlated with the concentration of TGF-β1 (p < 0.05).
Figure 3. Expression of α-SMA in mesangial cells after TGF-β1 induction. (a) Immunoblotting analysis of α-actin (upper gel) and of α-SMA (lower gel) protein expression 24 h co-culture with different concentrations of TGF-β1 (0, 1, 2, 5, and 10 ng/ml; o as control); (b) Representation of the densitometric intensity band ratio of α-SMA and α-actin that was used as internal control. **stands for p < 0.01.
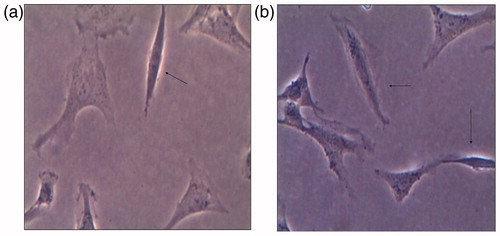
Morphological changes
Twelve-hour co-culture with 2 ng/mL of TGF-β1, only one or two mesangial cells presented with myofibroblast-like change (). 24 h co-culture with 2 ng/mL of TGF-β1 there were several mesangial cells that had myofibroblast-like change (). These indicate that TGF-β1 may induce a myofibroblast-like phenotype transformation.
Discussion
Premature senescence plays an important role in the process of organ fibrosis caused by a variety of diseases, such as acute ischemic/reperfusion injury,Citation10 diabetic nephropathy,Citation11 and chronic rejection of renal transplant.Citation12 Premature aging cells themselves do not only lose the proliferative capacity but also change the surrounding microenvironment by paracrine pathway, for example the secretion of IL-6, IL-8 and other mediators of inflammation, leading to chronic inflammation and fibrosis.Citation13,Citation14 Thus it should contribute to the early prevention and treatment of glomerulosclerosis to explore the effects of TGF-β1 on the cell cycle and phenotype of mesangial cells. This study adopted different concentrations and different durations of TGF-β1 to stimulate mesangial cells. The results demonstrated that TGF-β1 can induce the premature senescence and phenotype transformation of mesangial cells, and that the effect of TGF-β1 was time-dependent.
TGF-β1, a small molecular and soluble polypeptide, plays a very important role in the pathogenesis of glomerular sclerosis. TGF-β1 has high affinity receptors on mesangial cells, it can promote the hyperplasia, hypertrophy and proliferation of mesangial cells by binding to the receptors on mesangial cells. In addition, it can also promote the secretion of mesangial matrix, type I and type IV collagen through ERK1/2, PI3K and JNK-MAPK and other signal pathways, contributing to the development of glomerulosclerosis.Citation15,Citation16 The effects of TGF-β1 on mesangial cells are bidirectional. Low concentrations of TGF-β1 ( < 100 pg/mL) promote cell proliferation, high concentrations (>250 pg/mL) inhibit mesangial cell proliferation.Citation15 In consistent with the previous study, this current study proves again that high concentration of TGF-β1 (1 ∼ 10 ng/mL) inhibits the proliferation of mesangial cells. But this inhibition does not mean that there is no damage on mesangial cells. The damage may present with cells premature aging and/or hypertrophy.Citation17 In this current study, 15 min of induction of 1 ng/mL TGF-β1 made the cell cycle of mesangial cells arrested in G0/G1 phase and the cell cycle arrest is time-dependent. Therefore we think that it is credible that high concentration of TGF-β1 may cause the early damage of mesangial cells.
Cell phenotype transdifferentiation is an important reaction of cells to injury, it can lead to serious pathological states, such as vasculitis,Citation18 chronic rejection after renal transplantation,Citation19 diabetic nephropathyCitation20–22 and acute tubular injury caused by ischemia/reperfusion.Citation10 In pathological state, mesangial cells may swell and transform into myofibroblasts that express specific phenotypic marker α-SMA and meanwhile produce collagen (type I and type IV), glycoprotein (fiber connexin, laminin and actin) and proteoglycans in a large quantity. In addition, the myofibroblast-like phenotype transformed cells may secrete matrix metalloproteinase inhibitors to degrade the activity of matrix metalloproteinase, leading to the generation of mesangial extracellular matrix greater than its degradation and the excessive sedimentation in renal interstitium.Citation9 Study indicates that the mesangial cells with the expression of α-SMA have a strong contraction capacity and this may result in renal structure remodeling and glomerular ischemic sclerosis.Citation15 This current study demonstrated that 12 h of induction of TGF-β1 could make the phenotype of mesangial cells changed into myofibroblast, and with the time prolonged this phenotype transition occurred more and more, confirming that high concentration of TGF-β1 can lead to the damage of mesangial cells.
α-SMA is generally considered to be the iconic antigen of mesangial cells in active state.Citation9 Stephenson et al. found that α-SMA expression was up-regulated to at least 10 times higher 3–5 days after mesangial cells were co-cultured with 10 ng/mL TGF-β1 and meanwhile the cell volume of mesangial cells was enlarged. They thought that the expression of α-SMA reflected more the hypertrophy and hyperplasia of mesangial cells rather than the proliferation.Citation17 We agree to their opinion and our data indicated a positive correlation between the concentration of TGF-β1 and the α-SMA expression. These may suggest that the TGF-β1-induced premature aging and phenotype transition of mesangial cells is closely related to the early stage of glomerulosclerosis.
In summary, TGF-β1 may induce mesangial cells’ premature senescence and myofibroblast-like phenotype transformation time-dependently, which may contribute to the development of early stage of glomerulosclerosis.
Declaration of interest
No conflict of interest was declared.
This work was supported by grants from the National Natural Science Foundation of China (NO. 81100530, 81070590, 30801504, 30901926), Scientific and technological project of Shaanxi Province (NO. 2011K14-09-02, 2012K16-08-04).
References
- Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB J. 1987;1:272–281
- Jiao S, Meng F, Zhang J, Yang X, Zheng X, Wang L. STAT1 mediates cellular senescence induced by angiotensin II and H2O2 in human glomerular mesangial cells. Mol Cell Biochem. 2012;365:9–17
- Aihara K, Ikeda Y, Yagi S, Akaike M, Matsumoto T. Transforming growth factor-beta1 as a common target molecule for development of cardiovascular diseases, renal insufficiency and metabolic syndrome. Cardiol Res Pract. 2011;2011:175381 9 pp. doi: 10.4061/2011/175381.
- Desmouliere A, Darby IA, Gabbiani G. Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest. 2003;83:1689–1707
- Schnaper HW, Jandeska S, Runyan CE, et al. TGF-beta signal transduction in chronic kidney disease. Front Biosci. 2009;14:2448–2465
- Lopez-Novoa JM, Rodriguez-Pena AB, Ortiz A, Martinez-Salgado C, Lopez Hernandez FJ. Etiopathology of chronic tubular, glomerular and renovascular nephropathies: clinical implications. J Transl Med. 2011;9:13. 24 pp
- Hayashida T, Poncelet AC, Hubchak SC, Schnaper HW. TGF-beta1 activates MAP kinase in human mesangial cells: a possible role in collagen expression. Kidney Int. 1999;56:1710–1720
- Dorado F, Velasco S, Esparis-Ogando A, et al. The mitogen-activated protein kinase Erk5 mediates human mesangial cell activation. Nephrol Dial Transplant. 2008;23:3403–3411
- Kimura K, Iwano M. Molecular mechanisms of tissue fibrosis. Nihon Rinsho Meneki Gakkai Kaishi. 2009;32:160–167
- Lee DH, Wolstein JM, Pudasaini B, Plotkin M. INK4a deletion results in improved kidney regeneration and decreased capillary rarefaction after ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2012;302:F183–F191
- Verzola D, Gandolfo MT, Gaetani G, et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295:F1563–F1573
- Koppelstaetter C, Schratzberger G, Perco P, et al. Markers of cellular senescence in zero hour biopsies predict outcome in renal transplantation. Aging Cell. 2008;7:491–497
- Braun H, Schmidt BM, Raiss M, et al. Cellular senescence limits regenerative capacity and allograft survival. J Am Soc Nephrol. 2012;23:1467–1473
- Sis B, Tasanarong A, Khoshjou F, Dadras F, Solez K, Halloran PF. Accelerated expression of senescence associated cell cycle inhibitor p16INK4A in kidneys with glomerular disease. Kidney Int. 2007;71:218–226
- Sterzel RB, Schulze-Lohoff E, Marx M. Cytokines and mesangial cells. Kidney Int Suppl. 1993;39:S26–S31
- Das F, Ghosh-Choudhury N, Kasinath BS, Choudhury GG. TGFbeta enforces activation of eukaryotic elongation factor-2 (eEF2) via inactivation of eEF2 kinase by p90 ribosomal S6 kinase (p90Rsk) to induce mesangial cell hypertrophy. FEBS Lett. 2010;584:4268–4272
- Stephenson LA, Haney LB, Hussaini IM, Karns LR, Glass WF 2nd. Regulation of smooth muscle alpha-actin expression and hypertrophy in cultured mesangial cells. Kidney Int. 1998;54:1175–1187
- Bariety J, Hill GS, Mandet C, et al. Glomerular epithelial-mesenchymal transdifferentiation in pauci-immune crescentic glomerulonephritis. Nephrol Dial Transplant. 2003;18:1777–1784
- Bedi S, Vidyasagar A, Djamali A. Epithelial-to-mesenchymal transition and chronic allograft tubulointerstitial fibrosis. Transplant Rev (Orlando). 2008;22:1–5
- Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080
- Li J, Bertram JF. Review: Endothelial-myofibroblast transition, a new player in diabetic renal fibrosis. Nephrology (Carlton). 2010;15:507–512
- Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol. 2009;175:1380–1388