Abstract
Antiretroviral medications, specifically tenofovir, have been linked to acute tubular necrosis in humans with a suggested mechanism of direct tubular injury. Rhabdomyolysis has rarely been described in patients on highly active antiretroviral therapy (HAART). To the best of our knowledge, severe recurrent rhabdomyolysis-induced acute kidney injury (AKI) in a HIV-infected patient on two different triple antiretroviral regimens has not been reported. We present a HIV-positive patient who first developed heme pigment-induced oliguric AKI due to non-traumatic rhabdomyolysis, 5 days after initiation of triple antiretroviral therapy. Renal function normalized 2 months after discontinuation of antiretroviral therapy. Two weeks after reinitiating a different HAART regimen, our patient developed a recurrent episode of severe rhabdomyolysis-induced AKI. Both rhabdomyolysis and AKI resolved after discontinuation of the second antiretroviral regimen. First tenofovir and subsequently abacavir seem to be the likely culprits in our case. We also briefly discuss tenofovir nephrotoxicity followed by a literature review on rhabdomyolysis in HIV-infected patients.
Introduction
The International AIDS Society currently recommends instituting therapy for HIV-positive individuals when the CD4 count drops below 500/mm3.Citation1 As highly active antiretroviral therapy (HAART) is being instituted at a higher CD4 count, the side effects of these agents must be readily detectable by the clinician given that more HIV-positive patients will be on these medications. Initial antiretroviral regimens include at least three antiretroviral agents. One such preferred initial antiretroviral regimen consists of two nucleotide/nucleoside reverse-transcriptase inhibitors (NRTIs) plus a non-nucleoside reverse-transcriptase inhibitor (NNRTI). Current antiretroviral agents are associated with various adverse effects. One particular NRTI, tenofovir, has been associated with several renal manifestations including Fanconi syndrome, nephrogenic diabetes insipidus, as well as acute tubular necrosis (ATN), although the risk of acute kidney injury (AKI) is only ∼1%.Citation2
Rhabdomyolysis has rarely been reported with HAART. In a tolerability study of 166 patients that completed 30 days of HIV post-exposure prophylaxis with tenofovir/emtricitabine and lopinavir/ritonavir, there was only one instance of rhabdomyolysis.Citation3 This occurred in a 32-year-old female that presented with myalgias and elevated creatine kinase (CK) level, 10 days after initiating tenofovir/emtricitabine and lopinavir/ritonavir. However, AKI was not reported in this case. Mikhail et al.Citation4 also reported a HIV-positive patient on tenofovir that developed rhabdomyolysis 4 months after the dose of pravastatin was doubled from 40 to 80 mg.
Another NRTI, abacavir, has also been associated with an elevation of CK and myalgias in up to 6% of patients, coinciding with a hypersensitivity reaction.Citation5 AKI was not reported in this series. To the best of our knowledge, there is only one case of rhabdomyolysis reported in the setting of abacavir that was not associated with a hypersensitivity reaction. However, AKI was not reported in this case.Citation6
We present a unique case of an HIV-positive patient that developed severe recurrent rhabdomyolysis with AKI secondary to two different HAART regimens. Our patient did not have any other known risk factors for the development of rhabdomyolysis. First tenofovir and subsequently abacavir are likely to be the culprit drugs in this case.
Case presentation
A 42-year-old African-American man with HIV infection for 2 years presented to the emergency room with a 2-week history of right buttock and perineal pain. He complained of fever, chills, and generalized muscle aches as well as decreased urine output. He was diagnosed with a right gluteal and perineal abscess. Nephrology consultation was called for evaluation of elevated serum creatinine (7.38 mg/dL). The patient was also found to be oliguric. There was no history of any recent trauma or immobilization. There was no history of seizure disorder or any other significant past medical history. Medications on presentation included efavirenz, emtricitabine, and tenofovir, which were started 5 days prior to presentation. The patient denied statin, illicit drug, or alcohol use.
On physical exam, the patient was afebrile and normotensive. He had bilateral lower extremity pitting edema and a gluteal and perineal abscess. Urinalysis was significant for 3+ blood and 10–20 red blood cells per high power field. In addition to elevated serum creatinine level, initial laboratory evaluation revealed elevated serum transaminases (aspartate aminotransferase: 1174 U/L, alanine aminotransferase: 406 U/L) and elevated CK level of 199,000 U/L. Serum potassium and phosphorous were within normal limits.
On admission, the patient was initially started on intravenous (IV) fluids and IV antibiotics. His antiretroviral therapy was discontinued. There was no evidence of pyomyositis on computed tomography (CT) scan. The abscess was surgically drained. Blood cultures were negative, but the abscess was found to be positive for methicillin-resistant Staphylococcus aureus. The patient was initiated on hemodialysis for management of worsening oliguric AKI and hyperkalemia. Further laboratory evaluation revealed low serum complements (C3: 29.9 mg/dL, C4: 0.2 mg/dL) and negative anti-streptolysin O titers. HIV viral load was undetectable. A kidney biopsy was subsequently performed.
Kidney biopsy findings
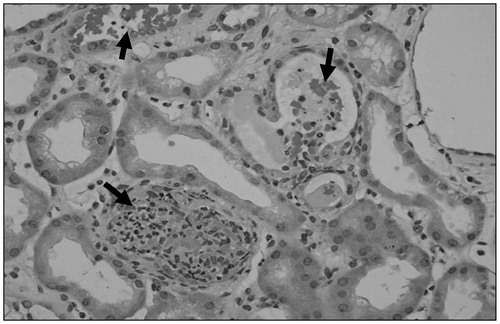
Light microscopy showed cellular and acellular casts in several tubular lumens (). Some of the tubules were obliterated by cellular casts. The casts were bright red with trichrome stain and were also strongly positive for myoglobin stain (immunohistochemistry method). Tubular casts were positive for myoglobin on immunoperoxidase staining. Attenuation of tubular epithelial cells was noted. The glomeruli were normal on light and electron microscopy and no immune deposits were seen on electron microscopy. Immunofluorescence was negative. The findings were consistent with ATN secondary to rhabdomyolysis.
Clinical follow-up
Our patient’s condition improved during the hospital stay. His AKI started to resolve and CK level normalized after 2 weeks. The patient required hemodialysis support for 2 weeks. Serum creatinine normalized after 2 months.
Four months after the first episode, the patient was restarted on efavirenz and emtricitabine and was also started on abacavir instead of tenofovir. Two weeks after reinitiating HAART, our patient was hospitalized with recurrent rhabdomyolysis (CK 233,000 U/L) and non-oliguric AKI. His serum creatinine on admission was elevated to 4.04 mg/dL. His serum transaminases were also elevated. Both renal function and CK levels improved after discontinuation of antiretroviral therapy and IV hydration.
Three months after the second episode, his creatinine had improved to 1.41 mg/dL and CK returned to the normal range. The patient was restarted on efavirenz and emtricitabine. Eleven months later, the patient’s serum creatinine remained stable at 1.3 mg/dL.
Discussion
Our patient with a 2-year history of HIV infection developed severe recurrent rhabdomyolysis-induced AKI while taking two different HAART regimens. The first episode of rhabdomyolysis occurred soon after initiation of HAART. The etiology of the AKI was initially confirmed with a kidney biopsy showing ATN with myoglobin casts. This excluded other etiologies of kidney injury, including acute interstitial nephritis and acute glomerulonephritis. After the first presentation, renal function normalized 2 months after discontinuing antiretroviral therapy. Our patient subsequently presented with severe AKI just 2 weeks after restarting an antiretroviral regimen consisting of abacavir, emtricitabine, and efavirenz. Although no kidney biopsy was done during the second presentation, the patient had severe rhabdomyolysis and no other possible cause of AKI could be identified. Initially we questioned whether emtricitabine and efavirenz also contributed to the rhabdomyolysis and the kidney injury, but now 11 months after restarting these two medications, the patient’s renal function has remained stable and he has had no further recurrences of rhabdomyolysis.
In humans, antiretroviral medications, especially tenofovir, are known to cause ATN with a suggested mechanism of tubular injury. The prodrug tenofovir disoproxil fumarate undergoes a hydrolysis reaction to form tenofovir, which is subsequently phosphorylated to the active drug tenofovir diphosphate. The active form is an inhibitor of HIV-1 reverse transcriptase and results in the early termination of DNA synthesis.Citation7 With a half-life of ∼17 h, tenofovir is eliminated via a combination of glomerular filtration and active tubular secretion. The human renal organic anion transporter 1 (hOAT1) is located in the proximal tubule on the basolateral side of the cell membrane. Tenofovir, and the other NRTIs as well, are taken up by this transporter and accumulate within the cells of the proximal tubule.Citation8,Citation9 A multidrug resistance-associated protein (MRP) is then involved with secretion of the drug into the tubule.Citation10,Citation11 Accumulation of a NRTI in the proximal tubule cells can result in nephrotoxicity via two potential mechanisms. The first involves tubular cell apoptosis caused by direct cytotoxicity of the drugCitation12 and the second involves inhibition of replication and eventual depletion of mitochondrial DNA from proximal tubular cells.Citation13,Citation14
Tenofovir, although less toxic than other NRTIs, does result in nephrotoxicity. There have been several animal studies showing that prolonged administration of high dose tenofovir results in clinical manifestations of the Fanconi syndrome including glucosuria, hyperphosphaturia, hypophosphatemia, and aminoaciduria. Pathology in these animal studies revealed interstitial nephritis and tubular atrophy.Citation15 A number of human case studies have documented AKI, diabetes insipidus, nephrolithiasis, and osteomalacia in those taking tenofovir. Nephrotoxicity is often divided into proximal tubular dysfunction with either normal or impaired renal function.Citation16
Zimmerman et al.Citation17 reviewed five patients from their institution that developed ATN while on tenofovir and performed a literature search and found an additional 22 patients with ATN. There was a mean increase in creatinine from 0.9 to 3.9 mg/dL. The co-administered drugs most often included ritonavir or lopinavir–ritonavir (21 of 27), didanosine (9 of 27) or atazanir (5 of 27). Two such patients required temporary dialysis. Biopsy in eight patients showed features consistent with ATN. Other pathology in these patients consisted of 16 patients with Fanconi syndrome, 19 with metabolic acidosis, 13 with hypokalemia, and 5 with nephrogenic diabetes insipidus. Once the tenofovir was discontinued, Fanconi syndrome was resolved in all patients, and renal function returned to baseline values in 22 of the 27 patients. The authors of the above study concluded that ritonavir likely increased the concentration of tenofovir in the proximal tubular cells via inhibition of MRP, thus causing toxicity.Citation17 Rhabdomyolysis, however, was not reported as a cause of AKI in this case series. It was initially believed that MRP 2 was the channel that caused tenofovir secretion, and because this channel is inhibited by ritonavir an accumulation of tenofovir would result.Citation17 Later studies have indicated that MRP 4 is the transporter channel through which tenofovir is secreted.Citation10 Because MRP 4 and not MRP 2 is used in tenofovir secretion, this mechanism does not completely reveal how renal toxicity develops. There is also an interaction between tenofovir and didanosine resulting in increased amounts of didanosine, which may compete with tenofovir for the hOAT1 transporter, resulting in increased risk for nephropathy and mitochondrial damage.Citation17
There have been discrepancies in the literature between clinical trials with the lack of observed tenofovir nephrotoxicity and case reports, in which adverse effects are reported. This is likely because of strict inclusion/exclusion criteria in the trials and the absence of real world comorbidities.Citation16
The main causes of rhabdomyolysis include crush injuries, burns, infections, medications, illicit drugs, and exercise. There are several causes of rhabdomyolysis in a patient with HIV infection. Joshi and LiuCitation18 reported a case series of seven HIV-positive patients that were admitted to an acute care hospital with rhabdomyolysis. Three of these patients developed significant AKI with a peak creatinine that ranged from 2.1 to 2.9 mg/dL. The need for renal replacement therapy was not reported in this study.Citation18 The risk factors most often attributed to rhabdomyolysis were infection, found in four patients, and substance abuse, present in six patients. The infectious agents included Staphylococcus aureus bacteremia in two patients, Pseudomonas aeruginosa bacteremia in one patient, and a Mycoplasma pneumoniae respiratory tract infection in one patient. Alcohol abuse, heroin, barbiturates, and cocaine were among the illicit substances abused. Other causes, including trauma or convulsions, accounted for the remaining risk factors. Three risk factors, on average, applied to each patient in the series. Zidovudine was the only antiretroviral therapy present, in use by two of the seven patients.Citation18 Our patient did not possess any of these above risk factors during both episodes except for localized infection during the first episode. There was no evidence of infective myositis in our patient on CT scan. Younger et al.Citation19 reported a case of recurrent myoglobinuria without AKI in a patient with HIV-associated polymyositis. Although myoglobulinuria can occur in patients with polymyositis, renal failure is rare. Our patient did not have any findings suggestive of polymyositis.
Our patient experienced recurrent episodes of rhabdomyolysis in the setting of two different antiretroviral regimens. Hence, rhabdomyolysis must be included in the differential in any patient with HIV that develops AKI while on antiretroviral therapy. In particular, those patients that are taking zidovudine, didanosine, or integrase inhibitors are particularly susceptible.Citation18,Citation20,Citation21 To the best of our knowledge, there is only one reported case of a 47-year-old HIV-positive female on HAART, including tenofovir, developing symptomatic rhabdomyolysis, evidence of proximal tubular dysfunction, and AKI.Citation22 However, this episode of AKI was not severe enough to necessitate hemodialysis. An asymptomatic rise in CK level in a HIV-infected patient taking tenofovir has also been reported,Citation23 but the previous case highlights a symptomatic presentation.
Abacavir hypersensitivity has been reported in 6% of patients on the drug, manifested by myalgias and CK elevation.Citation5 Other features of this condition include fever, constitutional symptoms such as malaise, dizziness, and headache, and gastrointestinal disturbances. A case of an African-American male with HIV infection who developed symptomatic rhabdomyolysis on abacavir has been previously reported.Citation6 Rhabdomyolysis resolved with discontinuation of HAART. AKI, however, did not occur in this case. That patient did not have any clinical manifestations of the hypersensitivity reaction, or any other obvious causes of rhabdomyolysis, such as HIV, infective or drug-induced myositis, malignancy, lactic acidosis or immune reconstitution syndrome. The authors concluded that abacavir was thus the likely culprit.Citation6
summarizes the reported cases of rhabdomyolysis that have been associated with tenofovir and/or abacavir in the literature.
Table 1. Reported cases of rhabdomyolysis that have been associated with tenofovir and/or abacavir in the literature.
Conclusion
In conclusion, we report a unique case of severe recurrent rhabdomyolysis-induced AKI in a HIV-infected patient on two different antiretroviral regimens. We believe that first tenofovir and then abacavir were responsible for rhabdomyolysis and AKI in our patient. Based on our experience, one should consider rhabdomyolysis in the differential diagnosis of AKI in a HIV-infected patient on antiretroviral therapy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
This case was presented as poster presentation at National Kidney Foundation 2012 Spring Clinical Meetings in Washington, DC, in May 2012. Dr. Louis R. Spiegel is a Nephrology Fellow at the Hofstra North Shore-LIJ School of Medicine. Dr. Hitesh H. Shah is the Director of the Nephrology Fellowship Program at the Hofstra North Shore-LIJ School of Medicine.
References
- Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304(3):321–333
- Szchzech LA. Renal dysfunction and tenofovir toxicity in HIV-infected patients. Top HIV Med. 2008;16(4):122–126
- Tosini W, Muller P, Prazuck T, et al. Tolerability of HIV postexposure prophylaxis with tenofovir/emtricitabine and lopinavir/ritonavir tablet formulation. AIDS. 2010;24(15):2375–2380
- Mikhail N, Iskander E, Cope D. Rhabdomyolysis in an HIV-infected patient on anti-retroviral therapy precipitated by high-dose pravastatin. Curr Drug Saf. 2009;4(2):121–122
- Hetherington S, McGuirk S, Powell G, et al. Hypersensitivity reactions during therapy with the nucleoside reverse transcriptase inhibitor abacavir. Clin Ther. 2001;23(10):1603–1614
- Parsonage MJ, Barlow G, Lillie P, et al. Severe myositis on commencement of efavirenz, abacavir and lamivudine, in the absence of lactic acidosis or classical abacavir hypersensitivity. BMJ Case Rep. 2009;2009. doi: 10.1136/bcr.01.2009.1411
- Physicians’ Desk Reference (Viread package insert). 61st ed. Montvale, NJ: Thompson PDR; 2007:1301–1308
- Ho ES, Lin DC, Mendell DB, Chilar T. Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J Am Soc Nephrol. 2000;11(3):338–393
- Cihlar T, Ho ES, Lin DC, Mulato AS. Human renal organic anion transporter 1 (hOAT1) and its role in nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids. 2001;20(4–7):641–648
- Ray AS, Cihlar T, Robinson KL, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother. 2006;50(10):3297–3304
- Servais A, Lechat P, Zahr N, et al. Tubular transporters OAT1 and MRP2 and clearance of adefovir. Nephrol Ther. 2005;1(5):296–300
- Ortiz A, Justo P, Sanz A, et al. Tubular cell apoptosis and cidofovir-induced acute renal failure. Antivir Ther. 2005;10(1):185–190
- Tanji N, Tanji K, Kambham N, et al. Adefovir nephrotoxicity: possible role of mitochondrial DNA depletion. Hum Pathol. 2001;32(7):734–740
- Bendele R, Richardson F. Adefovir nephrotoxicity and mitochondrial DNA depletion. Hum Pathol. 2002;33(5):574
- Van Rompay K, Brignolo L, Meyer D, et al. Biological effects of short term or prolonged administration of 9-[2-(phosponomethoxy)propyl]adenine (tenofovir) to newborn and infant rhesus macaques. Antimicrob Agents Chemother. 2004;48(5):1469–1487
- Cooper RD, Wiebe N, Smith N, et al. Systematic review and meta analysis: renal safety of tenofovir disoproxil fumarate in HIV infected patients. Clin Infect Dis. 2010;51(5):496–505
- Zimmerman AE, Pizzoferrato T, Bedford J, et al. Tenofovir associated acute and chronic kidney disease: a case of multiple interactions. Clin Infect Dis. 2006;42(2):283–290
- Joshi MK, Liu HH. Acute rhabdomyolysis and renal failure in HIV-infected patients: risk factors, presentation, and pathophysiology. AIDS Patient Care STDs. 2000;14(10):541–548
- Younger DS, Hays AP, Uncini A, et al. Recurrent myoglobinuria and HIV seropositivity: incidental or pathogenic association? Muscle Nerve. 1989;12(10):842–843
- Dori L, Buonomni AR, Viscione M, et al. A case of rhabdomyolysis associated with raltegravir use. AIDS. 2010;24(3):473–475
- Zembower TR, Gerzenshtein L, Coleman K, Palella FJ Jr. Severe rhabdomyolysis associated with raltegravir use. AIDS. 2008;22(11):1382–1384
- Callens S, De Roo A, Colebunders R. Fanconi-like syndrome and rhabdomyolysis in a person with HIV infection on highly active antiretroviral treatment including tenofovir. J Infect. 2003;47(3):262–263
- Shere-Wolfe KD, Verley JR. Marked elevation of the creatine phosphokinase level in a patient receiving tenofovir. Clin Infect Dis. 2002;35(9):1137