Abstract
Introduction: Type 1 (distal) renal tubular acidosis (RTA) is a rare clinical condition characterized with defect of urinary acidification in distal tubulus. If diagnosis delays, RTA may cause metabolic and clinical complications and comorbidities. We describe here a type 1 distal RTA case with symptoms mimicking coronary ischemia. Case report: A 46-year-old woman admitted with complaints of chest pain, palpitation, walking disability, fatigue and nausea. On physical examination muscles were weaken 3/5 in four extremities. An electrocardiogram revealed supraventricular tachycardia and ST depression on precordial V2-6 derivations. An acute coronary syndrome diagnosis made based on anginal symptoms, supraventricular tachycardia, ST depression on V2-6 derivations and elevated cardiac enzymes. Urgent coronary angiography was normal except a 30% narrowing in LAD. She had recurrent nephrolithiasis and had operated because of hydronephrosis. She had two episodes of fatigue and walking disability previously. Hyperchloremic metabolic acidosis with normal anion gap determined in blood gas analyze. Patient diagnosed with type I RTA with the signs and symptoms of recurrent nephrolithiasis, fatigue, severe hypokalemia (1.8 mmol/L), hyperchloremic metabolic acidosis with normal anionic gap, alkaline urine (pH 8) and positive urinary anionic gap (13.7 mmol/L). Sodium bicarbonate infusion and potassium replacement therapy administered. Clinical and laboratory signs of the patient dissolved during treatment. Conclusion: Type 1 RTA should be considered in acidotic patients admitted with hypokalemia and coronary symptoms. Urinary and blood gas analyses should be done beside cardiac tests initially. Therefore, a precise diagnosis may be possible without the possible complications of unnecessary coronary interventions.
Introduction
Type 1 (distal) renal tubular acidosis (RTA) is a rare clinical condition characterized with disability of urinary acidification in distal tubulus.Citation1 Defects in H-ATPase in luminal membrane or Cl–HCO3 exchanger in basolateral membrane, various drugs and systemic diseases may cause type 1 RTA.Citation2 Accompanied metabolic abnormalities may evoke the signs and symptoms seen in RTA cases. If diagnosis delays, RTA may cause metabolic and clinical complications and comorbidities.Citation1
We described here a RTA case with symptoms mimicking coronary ischemia.
Case report
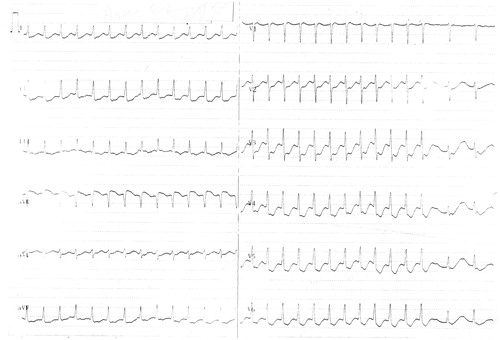
A 46-year-old woman admitted to emergency department with complaints of chest pain, palpitation, walking disability, muscle weakness in four extremities, fatigue and nausea. Her blood pressure was 110/70 mmHg, heart rate was 122/min, body temperature was 36.6°C and respiration rate was 22/min. On physical examination, Babinski reflexes were negligent bilaterally and muscles were weakening 3/5 in both upper and lower extremities. There were no other abnormalities on physical examination. An electrocardiogram revealed supraventricular tachycardia and ST depression on precordial V2-6 derivations (). Laboratory tests were as follows; urea: 39 mg/dL (10–50 mg/dL), creatinine: 1.45 mg/dL (0–1.5 mg/dL), Na: 141 mmol/L (135–148 mmol/L), Cl: 123 mmol/L (98–110 mmol/L), K: 1.8 mmol/L (3.5–5.5 mmol/L), Calcium: 9.2 mg/dL (8.0–10.4 mg/dL), CK: 290 U/L (0–190 U/L), CK-MB: 65.4 U/L (0–24 U/L) and troponin I < 0.20 ng/mL (0–0.16 ng/mL). An acute coronary syndrome diagnosis made in lights of anginal symptoms, supraventricular tachycardia, ST depression on V2-6 derivations and elevated cardiac enzyme markers (CK-MB). Urgent coronary angiography was normal except a 30% narrowing in LAD.
Patient has been transferred to nephrology clinic to evaluate metabolic disturbances. Detailed history obtained from the patient. She had recurrent nephrolithiasis and had operated because of hydronephrosis. She had two episodes of fatigue and walking disability previously. Hyperchloremic metabolic acidosis with normal anion gap determined in blood gas analyze (pH: 7.01, PaCO2: 36 mmHg, PaO2: 88 mmHg, bicarbonate: 6 mmol/L). Urinary pH was 8.0. Urinary sodium, potassium, and chloride were 68 mmol/L (15–237 mmol/L), 3.7 mmol/L (22–164 mmol/L), and 58 mmol/L (24–255 mmol/L), respectively. Urinary anion gap was 13.7 mmol/L reflecting impaired ammonium secretion. Acid loading test was not used for diagnosis because the patient had severe metabolic acidosis and a basic urine. Thyroid hormones, plasma renin, aldosterone, C3, C4, cryoglobulin and serum immunoglobulin levels were in normal range. Antinuclear antibodies, rheumatoid factor, anti-SSa (Ro), anti-SSb (La) were negative. Urinary ultrasound revealed enlargement (142 mm), certain cortical thinning and irregularities in right kidney. In addition, we detected a large number of dilated calices compatible with deforming cystic lesions and two urinary stones (9 mm ×2 mm and 6 mm × 3 mm) at the lower pole. Left kidney was normal in ultrasound scan. A bone mineral densitometry was negative for signs of osteopenia or osteoporosis. (AP spine, T score: 0.7, left femoral T score: −0.2.)
Patient diagnosed with type I RTA with the signs and symptoms of recurrent nephrolithiasis, fatigue, severe hypokalemia, hyperchloremic metabolic acidosis with normal anionic gap, alkaline urine and positive urinary anionic gap. Sodium bicarbonate infusion (2 meq/kg/day) and potassium replacement therapy (120 meq/day) administered. Metabolic electrolyte disturbances and blood gaze abnormalities dissolved at 48th hour of the therapy. A swelling and pain occurred in right inguinal region at third day of treatment. Right inguinal region was purple, sensitive to palpation and swollen. A computerized tomography scan revealed an hematoma sized 38 mm × 16 mm × 11 mm. The hematoma resorbed within 15 days. Clinical and laboratory signs of the patient dissolved and she discharged from hospital with oral sodium bicarbonate and potassium citrate. We noted in three clinic visits within 6 months after discharge from hospital that her clinical and laboratory signs remained normal.
Discussion
We describe here a type I RTA case mimicking coronary ischemia. Both severe hypokalemia (despite hyperchloremic metabolic acidosis with normal anion gap present) and alkaline urine with increased urinary anion gap were crucial to make the diagnosis.Citation3,Citation4 Recurrent nephrolithiasis and fatigue and muscle weakness were points of attention in medical history. We ruled out gastrointestinal bicarbonate loss and urinary infection with urease positive microorganisms.Citation3
RTA is a rare disorder. shows characteristic features and clinical types of RTA. Previous case reports published before about type I RTA was usually consistent of neurological symptoms.Citation5 Several reports were about quadriparesis related to distal RTA.Citation6 There is a strong correlation between the severity and development rate of hypokalemia and the neurological symptoms.Citation3,Citation7 It is even possible that type 1 RTA may present with more severe neurological symptoms such as respiratory paralysis.Citation8 According to the literature, both quadriparesis and walking disability were overt in our case. These neurological signs were associated with severe hypokalemia. There are type 1 RTA reports in literature that hypokalemia associated neurological signs contributed by thyrotoxicosis.Citation9 However, our case was both clinical and laboratory euthyroid. In other reports, hypokalemia related to type 1 RTA was found to be associated with autoimmune disorders, such as, systemic lupus erythematosus and Sjögren syndrome.Citation10,Citation11 We were unable to define such underlying disease in our case.
Table 1. Characteristic of different types of renal tubular acidosis (RTA).
Nephrolithiasis and nephrocalcinosis are common complications of distal type RTA.Citation12,Citation13 According to literature, our case had a history of urinary stone disease. Furthermore, ultrasound scan revealed destruction of renal parenchyma as a result of recurrent renal stones and hydronephrosis. These findings suggest that metabolic assessment should be done for patients with renal stone disease. A metabolic evaluation in time may prevent renal disease before irreversible destruction.
Osteopenia and osteoporosis are other complications of this rare clinical condition.Citation14 But they are more common in type 2 (proximal) RTA. In fact, we have not detected any bone mineral disorder in our case. These findings also help clinicians to rule out type 2 RTA in present case.
To our knowledge, there is no RTA case presenting with symptoms that mimic acute coronary syndromes. Patient had undergone urgent coronary angiography because symptoms on admission were correlated with acute coronary syndrome. Diagnosis made by nephrological assessment after coronary angiography failed to demonstrate any important coronary lesions. When the case investigated retrospectively, troponin I negativity despite CK-MB elevation should be considered as a hint of ruling out a coronary event. But electrocardiography changes accompanying chest pain and CK-MB elevation gave rise to an early acute coronary syndrome diagnosis that troponin I not elevated yet. Troponin I elevation occurs in 3–8th hour and reaches its peak within 24–48th hour of coronary event. Therefore, waiting for troponin I elevation may cause delay in both diagnosis and coronary revascularization and increase morbidity and mortality related to coronary ischemia.
In conclusion, type 1 RTA should be considered in acidotic patients admitted with hypokalemia and coronary symptoms. Recurrent renal stones and neurological signs should be questioned in history. Urinary and blood gas analyses should be done beside cardiac tests, initially. Cardiac enzyme elevations should be followed up and carefully monitored whether they reduce by treatment of both electrolyte disturbances and acid–base disorders. Therefore, a precise diagnosis may be possible without the possible complications of unnecessary coronary interventions.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Batlle D, Haque SK. Genetic causes and mechanisms of distal renal tubular acidosis. Nephrol Dial Transplant. 2012;27(10):3691–3704
- Han JS, Kim GH, Kim J, et al. Secretory-defect distal renal tubular acidosis is associated with transporter defect in H+-ATPase and anion exchanger-1. J Am Soc Nephrol. 2002;13(6):1425--1432
- Rose BD, Post TW. Clinical physiology of acidbase and electrolyte disorders. In: Rose BD, Post TW, eds. Metabolic acidosis. 5th ed. New York, NY: McGraw-Hill; 2001:578--646
- Kirschbaum B, Sica D, Anderson FP. Urine electrolytes and the urine anion and osmolar gaps. J Lab Clin Med. 1999;133(6):597–604
- Battista S, Urbino R, Antro C, Gai V. Acute paralysis due to distal renal tubular acidosis: a case report. Int Emerg Med. 2008;3(2):175–177
- Aygen B, Dursun FE, Dogukan A, Ozercan IH, Celiker H. Hypokalemic quadriparesis associated with renal tubular acidosis in a patient with Sjogren’s syndrome. Clin Nephrol. 2008;69(4):306–309
- Hattori N, Hino M, Ishihara T, Moridera K, Ikekubo K, Kurahachi H. Hypokalemic paralysis associated with distal renal tubular acidosis. Intern Med. 1992;31(5):662–665
- Vendeloo M, Aarnoudse ALHJ, van Bommel EFH. Life-threatening hypokalaemic paralysis associated with distal renal tubular acidosis. Neth J Med. 2011;69(1):35–38
- Im EJ, Lee JM, Kim JH, et al. Hypokalemic periodic paralysis associated with thyrotoxicosis, renal tubular acidosis and nephrogenic diabetes insipidus. Endocr J. 2010;57(4):347–350
- Koul P, Wahid A, Shah B. Systemic lupus erythematosus with distal renal tubular acidosis presenting as hypokalemic paralysis with respiratory failure. Saudi J Kidney Dis Transpl. 2003;14(2):190
- Soy M, Pamuk ON, Gerenli M, Celik Y. A primary Sjogren’s syndrome patient with distal renal tubular acidosis, who presented with symptoms of hypokalemic periodic paralysis: report of a case study and review of the literature. Rheumatol Int. 2005;26(1):86–89
- Goldfarb DS. A woman with recurrent calcium phosphate kidney stones. Clin J Am Soc Nephrol. 2012;7(7):1172–1178
- Arampatzis S, Ropke-Rieben B, Lippuner K, Hess B. Prevalence and densitometric characteristics of incomplete distal renal tubular acidosis in men with recurrent calcium nephrolithiasis. Urol Res. 2012;40(1):53–59
- Kawashima M, Amano T, Morita Y, Yamamura M, Makino H. Hypokalemic paralysis and osteomalacia secondary to renal tubular acidosis in a case with primary Sjögren’s syndrome. Mod Rheumatol. 2006;16(1):48–51