Abstract
The association between angiotensin II type 1 receptor (AT1R) A 1166C (rs5186) gene polymorphism and end-stage renal disease (ESRD) risk remains controversial. We aimed to assess the association between AT1R A1166C gene polymorphism and ESRD susceptibility by performing a meta-analysis. Eligible studies were searched according to a predefined criterion using electronic databases. Eight articles were identified for the analysis of the association between AT1R A1166C gene polymorphism and ESRD risk. A allele and AA genotype were not associated with ESRD risk in overall populations, Caucasians and Asians (overall populations: p = 0.834 and 0.832, Caucasians: p = 0.853 and 0.884, Asians: p = 0.243 and 0.982). CC and AC genotype were not associated with ESRD risk in overall populations, Caucasians and Asians (overall populations: p = 0.304 and 0.712, Caucasians: p = 0.510 and 0.987, Asians: p = 0.319 and 0.225). In conclusion, AT1R A1166C gene polymorphism may not be correlated with ESRD risk in overall populations, Caucasians and Asians. However, more studies should be performed in the future.
Introduction
End-stage renal disease (ESRD), an entity with a continuing loss of endogenous renal function, is a public health problem across the world. Patients with ESRD have markedly elevated morbidity and mortality.Citation1 ESRD is a multifactorial disease, many risk factors are involved in the pathogenesis of ESRD. Increased genomic damage was observed in patients suffering from ESRD, and DNA repair gene polymorphisms may affect DNA repair capacity and modulate susceptibility to ESRD.Citation2 Genetic factors might be associated with the risk of ESRD and might predict its onset.Citation3,Citation4
It is well-documented that the renin–angiotensin system (RAS) is implicated in the pathogenesis of progressive renal disease including ESRD.Citation5,Citation6 The action of RAS is mediated primarily by angiotensin II.Citation7 Angiotensin II is a powerful vasoconstrictor and mediator of cellular proliferation, extracellular matrix protein synthesis, and accumulation.Citation8 The angiotensin II type 1 receptor (AT1R) is the primary pathogenic effector for angiotensin II.Citation9 In this sense, it is reasonable to postulate the mutation of gene encoding AT1R is involved in the etiology of ESRD. AT1R A1166C (rs5186) gene polymorphism, an important mutation of AT1R, has been investigated in a number of studies for its association with ESRD risk.Citation10–16 However, the results were in conflict. Meta-analysis of the association between AT1R A1166C gene polymorphism and ESRD risk was rare. Thus, we performed this meta-analysis to investigate the correlation between AT1R A1166C gene polymorphism and ESRD susceptibility with the aim of providing a much more reliable finding on the significance of the association.
Materials and methods
Search strategy
The published papers that tested the association between AT1R gene polymorphism and the risk of ESRD in humans were searched from March 2013 using Pubmed, Embase, and Cochrane databases. No restriction was imposed on search language. The used search terms were as follows: (1) end-stage renal disease, ESRD, renal failure, kidney failure; (2) angiotensin II type 1 receptor, AT1R, gene polymorphism, A1166C. We also reviewed the reference lists of retrieved articles and reviews. If the same participants were enrolled in more than one study, we chose the study with the most complete analysis.
Inclusion and exclusion criteria
Inclusion criteria
(1) A case-control study; (2) The outcome of interest was ESRD; (3) A minimum of two comparison groups (ESRD group vs. control group).
Exclusion criteria
(1) Case reports, reviews and editorials; (2) Association of other genes with ESRD risk; (3) Multiple publications of the same data; and (4) Investigation of the role of AT1R to diseases.
Data extraction and synthesis
We extracted study characteristics from each study. Data were recorded as follows: first author’s last name, year of publication, ethnicity of study population, number of cases and controls for AT1R A1166C genotype. Frequencies of A allele were calculated for case and control groups, from the corresponding genotype distribution. Two authors independently performed the data extraction with any disagreements resolved by discussion.
Statistical analysis
Odds ratio (OR) was used as a measure of the association between AT1R gene polymorphism and ESRD risk across studies. Heterogeneity of ORs among studies was tested using the Q statistic (significance level at p < 0.10). The I2 statistic, a quantitative measure of inconsistency across studies, was also calculated. The combined ORs were calculated using either a fixed-effects model or, in the presence of heterogeneity, a random-effects model. Furthermore, 95% confidence intervals (CIs) were also calculated. The OR was calculated by using five methods: method 1, log-additive/per-allele model analysis; method 2, allele comparison (A allele vs. C allele); method 3, comparing AA homozygous with the other two combinations (AA vs. AC + CC); method 4, comparing CC genotype with the other two combinations (CC vs. AC + AA); and method 5, comparing AC genotype with the other two combinations (AC vs. AA + CC). A chi-squared test using a web-based program was used to determine whether genotype distribution of the control population reported conformed to Hardy–Weinberg equilibrium (HWE) (p < 0.05 was considered significant). Sensitivity analysis was performed when studies with controls were not in HWE. Meta-regression was performed to identify whether effect estimate was associated with age at baseline, male/female ratio or ethnicity. Potential publication bias was assessed by Begg’s test and Egger’s test at the p < 0.05 level of significance when the number of the included studies was more than five. All analyses were performed using STATA version 12.0 (Stata Corp, College Station, TX). p < 0.05 was considered statistically significant, except where otherwise specified.
Results
Study characteristics
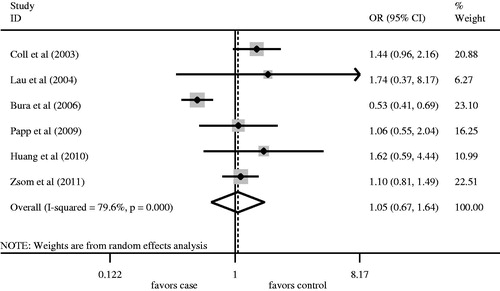
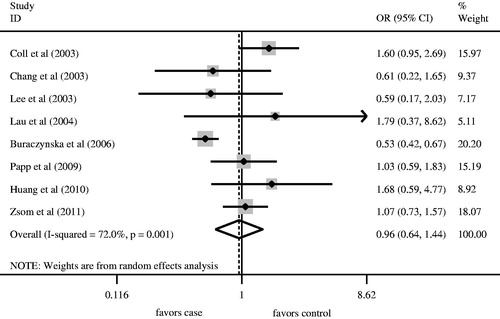
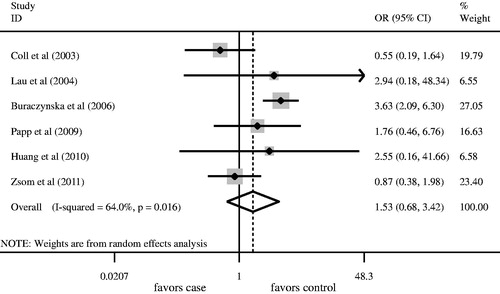
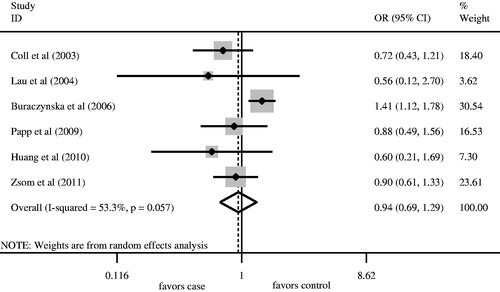
We firstly retrieved 573 unique citations from PubMed, Embase, and Cochrane databases. Of these, 565 publications were excluded according to the inclusion and exclusion criteria. Eight articles were enrolled in our analysis for the association between AT1R A1166C gene polymorphism and ESRD risk (). Four studiesCitation8,Citation10,Citation11,Citation13 were conducted in Caucasians, and fourCitation12,Citation14–16 in Asians. All articles were published in English. The data of interest were retrieved as follows: first author’s last name, year of publication and the number of cases and controls (). These eight studies included 1360 cases and 1449 controls. The average frequency of the AT1R A1166C A allele was 70.96% in Caucasian patients and 74.32% in controls. For Asians, the average frequency of A allele was 95.57% in the case group and 92.99% for controls. The ratio of cases/controls for average frequency of A allele in Caucasians was notably lower compared with that in Asians (Caucasians: cases/controls = 0.95; Asians: cases/controls = 1.03).
Table 1. Characteristics of studies evaluating the effects of AT1R A1166C gene polymorphism on ESRD risk.
Association of the AT1R A1166C gene polymorphisms with ESRD risk
Angiotensin II type 1 receptor A1166C gene polymorphisms were not associated with ESRD risk for Caucasians using the log-additive model (). No significant association between A allele/AA genotype and ESRD risk was observed in overall populations ( and , ). Meanwhile, the CC/AC genotype was not associated with ESRD risk ( and , ). In order to assess the racial effect, we stratified the populations by ethnicity. There was no marked relationship between AT1R A1166C gene polymorphism and ESRD risk in Caucasians and Asians ().
Table 2. Meta-analysis of the association of AT1R A1166C gene polymorphism with ESRD risk.
Meta-regression analysis
None of the covariates including age, male/female ratio and ethnicity demonstrated significant influences on the ORs (as shown in ).
Table 3. Characteristics of univariate meta-regression analysis.
Sensitivity analysis
Hardy–Weinberg equilibrium was unavailable in one studyCitation12 in which the detailed number of AC and CC genotype in the control group was not provided. The genotype distributions of the control groups in the remaining studies were all in HWE. Therefore, we recruited seven studiesCitation8,Citation10,Citation11,Citation13–16 into the sensitivity analysis. The results regarding the association between AA genotype and ESRD risk in overall populations, Caucasians and Asians were all similar to those in the non-sensitivity analysis ().
Assessment of publication bias
Visual inspection of funnel plot showed no significant asymmetry (). No significant publication bias for overall populations was observed (A vs. C: Begg p = 0.38, Egger p = 0.234; AA vs. AC + CC: Begg p = 0.12, Egger p = 0.142; CC vs. AA + AC: Begg p = 0.75, Egger p = 0.465; AC vs. AA + CC: Begg p = 0.851, Egger p = 0.057).
Discussion
AT1R A1166C gene polymorphism is due to a substitution of cytosine for adenine at the position 1166 in 3′ untranslated region.Citation17 It might be linked to an unidentified functional mutation in the AT1R gene or in another closely linked gene possibly located in regulatory regions, which is involved in the development and progression of renal damage.Citation8
Increasing attention has been paid to the association between AT1R A1166C gene polymorphism and ESRD risk. However, our meta-analysis indicated there was no significant relationship between AT1R A1166C gene polymorphism and ESRD risk in overall populations. The AA/AC/CC genotype demonstrated no relationship with the onset of ESRD, a similar result was observed in the A allele. The results from sensitivity analysis did not differ obviously from those from the non-sensitivity analysis. No evidence of publication bias in overall populations was observed, which indicated the results for overall populations were robust.
The ethnic differences might affect the association between AT1R A1166C gene polymorphism and ESRD risk. In our investigation, we observed that the average frequency of A allele in controls was 74.32% and 92.99% in Caucasians and Asians, respectively. The ratio of cases/controls for average frequency of A allele was 0.95 and 1.03 in Caucasians and Asians, respectively. Subgroup analysis was performed to investigate the association of AT1R A1166C gene polymorphism with the susceptibility of ESRD in different ethnicities.
In Caucasians, we observed no association between AT1R A1166C gene polymorphism and the susceptibility of ESRD. The genotype distributions of the control groups in all studies included were in HWE. Notably, there were only two studies enrolled investigating the association between A allele and ESRD risk, which limited its statistical power resulting in a less robust result. More studies should be conducted in the future.
Although a higher ratio of cases/controls for average frequency of A allele was observed in Asians (cases/controls = 1.03), no marked association between AT1R A1166C gene polymorphism and the susceptibility of ESRD was found. Meanwhile, the results from the sensitivity analysis were similar to those from the non-sensitivity analysis. Additionally, there were only six studies included investigating the association between AT1R A1166C gene polymorphism and ESRD risk, which made the conclusions less robust. A larger number of studies are warranted in the future.
In the past, the association between AT1R A1166C gene polymorphism and various diseases was explored. Zhang et al.Citation18 reported that A1166C allele and AC/CC genotypes were positively associated with pregnancy hypertensive disorders, particularly in Asians. Feng et al.Citation9 found that C allele is a risk factor for myocardial infarction and AA genotype might be a protective factor. Zhang et al.Citation19 reported that the C allele and AC/CC genotype increased the risk of coronary heart disease. Ding et al.Citation20 found that CC genotype might contribute to the development of diabetic nephropathy, particularly in type 2 diabetes mellitus. Conversely, Zhang et al.Citation21 reported no association between A1166C gene polymorphism and susceptibility to ischemic stroke. Xu et al.Citation22 found a weak association between A1166C gene polymorphism and susceptibility to coronary heart disease. Hunley et al.Citation23 found there was no difference between non-progressive renal disease and progressive renal disease. Pei et al.Citation24 observed that A1166C gene polymorphism was not associated with progression or proteinuria in IgA nephropathy. Frimat et al.Citation25 reported that A1166C gene polymorphism had no predictive value for renal survival in ESRD.
In our investigation, we found AT1R A1166C gene polymorphism was not associated with the risk of ESRD. Several factors may explain the uncertain relationship. First, multiple genes are involved in the activity regulation of the renin–angiotensin system (RAS), other genes and polymorphisms have been reported to contribute to RAS activity.Citation26,Citation27 The possible interaction between RAS gene polymorphisms might affect the development and progression of cardiovascular and renal diseases. Such interactions were observed between ACE I/D (rs4646994) and AGT M235T (rs699)/AT1R polymorphism. Second, the comorbidities, such as hypertension and diabetes would influence the expression levels of AT1R, which might be associated with A1166C gene polymorphism. Finally, the medications including ACE inhibitors and AT1R antagonists used in patients, might affect the results.
In our study, several limitations should be considered. First, heterogeneities might be present, affecting the results of our meta-analysis, although a random-effects model had been conducted. Although the meta-regression analysis showed no marked impact of age, male/female ratio and ethnicity on the pooled ORs, the potential influencing factors should be explored in the future. Second, the reporting bias was unavoidable. Finally, the small sample sizes, particularly in the subgroup analysis, limited the statistical power. The results should be interpreted with great caution.
In conclusion, the results of our study suggest that there an association between AT1R A1166C gene polymorphism and the susceptibility to ESRD in overall populations, Caucasians and Asians. However, more well-designed, stratified, case-control studies are required to clarify the role of AT1R gene polymorphism in ESRD risk of different ethnicities.
Declaration of interest
There is no conflict of interest for all authors. This study was supported by a grant of Research and innovation Project for College Graduates of Jiangsu Province, China (grant number CXLX13_556).
References
- Marson BP, de Figueiredo CE, Tanus-Santos JE. Imbalance matrix metalloproteinases in cardiovascular complications of end-stage kidney disease: a potential pharmacological target. Basic Clin Pharmacol Toxicol. 2012;110:409–415
- Trabulus S, Guven GS, Altiparmak MR, et al. DNA repair XRCC1 Arg399Gln polymorphism is associated with the risk of development of end-stage renal disease. Mol Biol Rep. 2012;39:6995–7001
- Prakash S, Prasad N, Sharma RK, et al. Vascular endothelial growth factor gene polymorphisms in North Indian patients with end stage renal disease. Cytokine. 2012;58:261–266
- Borkar M, Tripathi G, Sharma RK, et al. Chemokine (CCR) and fractalkine (CX3CR) receptors and end stage renal disease. Inflamm Res. 2011;60:399–407
- Alvarez R, Requero JR, Batalla A, et al. Angiotensin-converting enzyme and angiotensin II receptor 1 polymorphisms: association with early coronary disease. Cardiovasc Res. 1998;40:375–379
- Pontremoli R, Ravera M, Viazzi F, et al. Genetic polymorphism of the renin-angiotensin system and organ damage in essential hypertension. Kidney Int. 2000;57:561–569
- Timmermans PB, Wong PC, Chiu AT, et al. Angiotensin II receptors and angiotension II receptor antagonists. Pharmacol Rev. 1993;45:205–251
- Buraczynska M, Ksiazek P, Drop A, et al. Genetic polymorphisms of the renin-angiotensin system in end-stage renal disease. Nephrol Dial Transplant. 2006;21:979–983
- Feng X, Zheng BS, Shi JJ, et al. A systematic review and meta-analysis of the association between angiotensin II type 1 receptor A1166C gene polymorphism and myocardial infarction susceptibility. J Renin Angiotensin Aldosterone Syst. 2012 Nov;e0:1--9
- Zsom M, Fulop T, Zsom L, et al. Genetic polymorphisms and the risk of progressive renal failure in elderly Hungarian patients. Hemodial Int. 2011;15:501–508
- Coll E, Campos B, Nunez DG, et al. Association between the A1166C polymorphism of the angiotensin II receptor type 1 and progression of chronic renal insufficiency. J Nephrol. 2003;16:357–364
- Chang HR, Cheng CH, Shu KH, et al. Study of the polymorphism of angiotensinogen, anigiotensin-converting enzyme and angiotensin receptor in type II diabetes with end-stage renal disease in Taiwan. J Chin Med Assoc. 2003;66:51–56
- Papp F, Friedman AL, Bereczki C, et al. Renin-angiotensin gene polymorphism in children with uremia and essential hypertension. Pediatr Nephrol. 2003;18:150–154
- Lau YK, Woo KT, Choong HL, et al. Renin-angiotensin system gene polymorphisms: its impact on IgAN and its progression to end-stage renal failure among Chinese in Singapore. Nephrol Physiol. 2004;97:1–8
- Huang HD, Lin FJ, Li XJ, et al. Genetic polymorphisms of the renin–angiotensin–aldosterone system in Chinese patients with end-stage renal disease secondary to IgA nephropathy. Chin Med J. 2010;123:3238–3242
- Lee KB, Kim UK. Angiotensinogen and angiotensin II type 1 receptor gene polymorphism in patients with autosomal dominant polycystic kidney disease: effect on hypertension and ESRD. Yonsei Med J. 2003;44:641–647
- Bonnardeaux A, Davies E, Jeunemaitre X, et al. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension. 1994;24:63–69
- Zhang L, Yang H, Qin H, Zhang K. Angiotensin II type I receptor A1166C polymorphism increases the risk of pregnancy hypertensive disorders: evidence from a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2012 Dec;e0:1--8
- Zhang K, Zhou B, Zhang L. Association study of angiotensin II type 1 receptor: A1166C (rs5186) polymorphism with coronary heart disease using systematic meta-analysis. J Renin Angiotensin Aldosterone Syst. 2013;14:181--188
- Ding W, Wang F, Fang Q, et al. Association between two genetic polymorphisms of the renin-angiotensin-aldosterone system and diabetic nephropathy: a meta-analysis. Mol Biol Rep. 2012;39:1293–1303
- Zhang H, Sun M, Sun T, et al. Association between angiotensin II type 1 receptor gene polymorphism and ischemic stroke: a meta-analysis. Cerebrovasc Dis. 2011;32:431–438
- Xu M, Sham P, Ye Z, et al. A1166C genetic variation of the angiotensin II type 1 receptor gene and susceptibility to coronary heart disease: collaborative of 53 studies with 20435 cases and 23674 controls. Atherosclerosis. 2010;213:191–199
- Hunley TE, Julian BA, Phillips JA, et al. Angiotensin converting enzyme gene polymorphism: potential silencer motif and impact on progression in IgA nephropathy. Kidney Int. 1996;49:571–577
- Pei Y, Scholey J, Thai K, et al. Association of angiotensinogen gene T235 variant with progression of immunoglobin A nephropathy in Caucasian patients. J Clin Inves. 1997;100:814–820
- Frimat L, Philippe C, Maghakian MN, et al. Polymorphism of angiotensin converting enzyme, angiotensinogen, and angiotensin II type 1 receptor genes and end-stage renal failure in IgA nephropathy: IGARAS-a study of 274 men. J Am Soc Nephrol. 2000;11:2062–2067
- Lau YK, Woo KT, Choong HL, et al. ACE gene polymorphism and disease progression of IgA nephropathy in Asians in Singapore. Nephron. 2002;91:499–503
- Kim SM, Chin HJ, Oh YK, et al. Blood pressure-related genes and the progression of IgA nephropathy. Nephron Clin Pract. 2009;113:301–308