Abstract
Introduction: Epicardial adipose tissue (EAT) is the true visceral fat depot of the heart. The relationship between coronary artery disease and EAT was shown in end-stage renal disease (ESRD) patients. One of the established risk factor in this population is dyslipidemia. We aimed to determine the relationship between atherogenic index of plasma (AIP) and EAT in ESRD patients. Methods: This was a cross-sectional study involving 76 ESRD patients receiving PD or HD for ≥6 months and 42 healthy subjects. EAT was measured by using an electrocardiogram-gated 64-multidetector computed tomography (MDCT). Atherogenic index of plasma was calculated as the logarithmically transformed ratio of the serum trigliseride to HDL-cholesterol. Results: The etiology of ESRD patients was diabetic nephropathy (n = 16), chronic glomerulonephritis (n = 10), hypertensive nephropathy (n = 23), polycystic kidney disease (n = 7), nephrolithiasis (n = 5) and unknown (n = 15). There were no differences with respect to the following variables between ESRD patients and healthy subjects: age; sex; BMI; predialysis levels of DBP; serum levels of albumin, HDL-cholesterol and hemoglobin. However, ESRD patients had higher serum levels of trigliseride, hs-CRP and AIP when compared to healthy subjects. There was a statistically significant relationship between EAT, BMI and AIP in ESRD patients (r = 0.42, p < 0.001 and r = 0.25, p = 0.028, respectively). The stepwise linear regression analysis revealed that age, as well as BMI were independent predictors of EAT. Conclusion: We found a relationship between EAT as defined by MDCT and AIP in ESRD patients. Further clinical and experimental studies are needed.
Introduction
Dyslipidemia has been established as a well-known traditional risk factor for cardiovascular disease (CVD) in the general populationCitation1 and chronic kidney disease (CKD) patients receiving hemodialysis and peritoneal dialysis.Citation2 Large-scale observational studies have confirmed that dyslipidemia may actively participate in the pathogenesis of atherosclerosis in general population.Citation3 Additionally, it has been well known that CKD patients exhibit significant alterations in lipoprotein metabolism, which may result in the development of severe dyslipidemia in this population.Citation4 Previous studies demonstrated that hypertriglyceridemia might be the earliest laboratory finding among the other lipid abnormalities even in patients who have slightly elevated creatinine levels.Citation4,Citation5 In contrast, high density lipoprotein (HDL) cholesterol was found to inversely related to the cardiovascular risk in non-CKD population.Citation6 End-stage renal disease (ESRD) patients usually have increased concentrations of triglyceride-rich lipoproteins and reduced serum levels of HDL-cholesterol. However, low-density-lipoprotein (LDL) cholesterol values were found to be within normal limits or reduced in this population.Citation7
Epicardial adipose tissue (EAT) originates from the splanchnopleuric mesoderm.Citation8 Mazurek et al.Citation9 concluded that, like abdominal visceral adipose tissue, EAT is also metabolically active because it can secrete proinflammatory cytokines and utilize free fatty acids (FFAs). In a recent study, the authors demonstrated that EAT acts an extremely active organ that produces several bioactive adipokines, as well as proinflammatory and proatherogenic cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-6, resistin, visfatin, omentin, leptin, plasminogen activator inhibitor-1 (PAI-1) and angiotensinogen.Citation9–13 We recently showed that EAT is increased in HD and PD patientsCitation14,Citation15 and this active visceral fat tissue is closely related with malnutrition-inflammation-atherosclerosis/calcification syndrome (MIAC) in this population.Citation16
Logarithmic ratio of triglycerides to HDL was defined as atherogenic index of plasma (AIP) and this index was found to be closely associated with atherosclerosis in general population.Citation17 However, in the literature, data regarding the AIP in ESRD patients are scant. Hence, we aimed to investigate the relationship between AIP and EAT in ESRD patients receiving hemodialysis (HD) and peritoneal dialysis (PD) and to compare these results with the others that obtained from healthy subjects.
Study population and methods
This was a cross-sectional study involving 76 ESRD patients (30 females, 46 males; mean age, 49.5 ± 14 years) receiving PD or HD for ≥6 months in the Dialysis Unit of Selcuk University and 42 healthy control subjects (19 females, 23 males; mean age, 51 ± 12 years) between February and June 2009.
Patients aged 18–70 years willing to participate in the assessment of EAT by multidetector computed tomography (MDCT) were screened. A review of medical records (including information on age; sex; weight; duration of renal replacement treatment; medications; primary disease of ESRD) was undertaken. Exclusion criteria were: (i) congestive heart failure; (ii) active infection; (iii) autoimmune disease; (iv) secondary hyperparathyroidism; (v) nephrotic-range proteinuria. Ninety patients were evaluated and 14 patients were excluded from the study. Of these 14 patients, 4 patients had congestive heart failure (New York Heart Association (NYHA) class III–IV); 4 patients had active infection; 3 patients had secondary hyperparathyroidism; and 3 patients had autoimmune disease (including systemic lupus erythematosus and microscopic polyangiitis). None of the patients included in the study had nephrotic-range proteinuria and arrhythmia based on electrocardiography (ECG). The remaining 76 ESRD patients fulfilled the above criteria and were enrolled in the study. Forty-two age-matched and sex-matched healthy individuals referred from outpatient clinics of the Internal Medicine Department of Selcuk University were also enrolled as control subjects. They were subject to the same inclusion and exclusion criteria as the patients. HD patients were receiving thrice-weekly dialysis for a 4-h period with a standard bicarbonate-containing dialysate bath using a biocompatible HD membrane (Polysulfone, FX-80 series, Fresenius, Germany). Dialysate flow rates were 500 mL/min and blood-flow rates were 250–300 mL/min. The systolic blood pressure (SBP) and diastolic blood pressure (DBP) of patients and healthy subjects was measured in the upright sitting position after ≥5 min of rest using an Erka sphygmomanometer (PMS Instruments Limited, Berkshire, UK) with an appropriate cuff size. Two readings were recorded for each individual. The mean value of two readings was defined as the blood pressure. Patients with SBP and DBP >140 mmHg and 90 mmHg, respectively, or who were already on antihypertensive treatment were assumed to be hypertensive.
Twenty-three patients were taking antihypertensive drugs (14 of them on angiotensin-converting enzyme (ACE) inhibitors; 8 receiving angiotensin-II receptor blockers (ARB); and 1 receiving a calcium-channel blocker and an ACE inhibitor). Thirty-two patients were taking calcium-containing phosphate binders.
The study protocol was approved by the Medical Ethics Committee of Selcuk University (Meram School of Medicine, Konya, Turkey). Written informed consent was obtained from all subjects included in the study.
Biochemical analyses
Venous blood samples for biochemical analyses were drawn after an overnight fast before first exchange in PD patients and before the midweek session in patients receiving HD. All biochemical analyses including those for glucose, creatinine, total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol and plasma triglyceride (TG) concentrations were undertaken using an oxidase-based technique by the Roche/Hitachi Modular System (Mannheim, Germany) in the Central Biochemistry Laboratory of the Meram School of Medicine.
Definition of atherogenic index of plasma
Atherogenic index of plasma was calculated as the logarithmically transformed ratio of the serum triglyceride to HDL-cholesterol.Citation18
Evaluation of EAT
Unenhanced CT was quantified on retrospectively ECG-gated cardiac computed tomography (CT) using 64-slice multidetector computed tomography (MDCT; Sensation 64 Siemens Medical Solutions, Erlangen, Germany). The CT protocol was: slice collimation, 64 × 0.6 mm; gantry rotation time, 0.33 s; pitch, 0.2; tube voltage, 120 kV; and tube current, 600 mAs. If the heart rate (HR) was >65 beats per minute (bpm), HR control was achieved with a beta-blocker.
All images analysis was performed on a dedicated workstation (Leonardo Workstation Siemens Medical Solutions Erlangen, Germany) as previously described.Citation19 Adipose tissue quantification was performed in a semi-automated method that required manual definition of borders. CT attenuation thresholds were used to identify fax pixels (window width −195 to −45 HU; window center −120 HU) to calculate adipose volumes.
Statistical analyses
Statistical analyses were carried out using the Statistical Package for Social Sciences for Windows version 17.0 (SPSS, Chicago, IL). Parametric data are expressed as the mean ± SD. Dichotomous variables were compared using the chi-square test. Statistical differences between parametric data of two groups were analyzed using the Student’s t-test. The Mann–Whitney U test was used to determine differences between non-parametric data. Linear associations between continuous variables were assessed using the Spearman correlation test.
Multivariate linear regression analyses were undertaken to determine independent associations among EAT and other variables. Age, body mass index, AIP, serum LDL, hs-CRP, albumin and dialysis vintage were entered into the regression model as independent variables, and EAT was entered as a dependent variable. The backward elimination method was preferred in the stepwise regression analysis and p > 0.1 used as a criterion for elimination in this model. At the end of the sixth step, two variables were remained statistically significant in the model. p < 0.05 was considered significant for all tests.
Results
Baseline characteristics of patients
The baseline characteristics of 76 ESRD patients and 42 healthy subjects are shown in . The etiology of ESRD patients was diabetic nephropathy (n = 16), chronic glomerulonephritis (n = 10), hypertensive nephropathy (n = 23), polycystic kidney disease (n = 7), nephrolithiasis (n = 5) and unknown (n = 15). There were no differences with respect to the following variables between ESRD patients and healthy subjects: age; sex; BMI; predialysis levels of DBP; serum levels of albumin, HDL-cholesterol and hemoglobin. However, ESRD patients had higher serum levels of triglyceride, hs-CRP and AIP when compared to healthy subjects ().
Table 1. Demograhic and biochemical parameters of ESRD patients and healthy subjects.
Evaluation of epicardial adipose tissue
Total EAT volume was significantly higher in ESRD patients compared with healthy subjects (160 ± 76 cm3 vs. 122 ±38 cm3, p = 0.04, respectively). When patients were classified according to their median value of EAT as Group1, EAT <136 cmCitation3 and Group 2, EAT ≥ 136 cm3, only AIP levels were found to be statistically significant between two groups (0.65 ±0.25 vs. 0.49 ± 0.27, p = 0.048, respectively) ().
Table 2. Demographic features and biochemical values of ESRD patients according to EAT group.
Correlation analysis between EAT and other variables
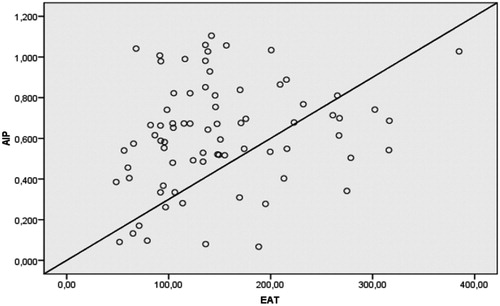
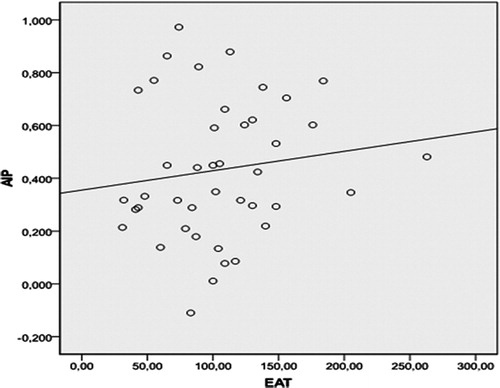
There was a statistically significant relationship between EAT, BMI and AIP in ESRD patients (r = 0.42, p < 0.001 and r = 0.25, p = 0.028, respectively). The correlation between EAT and AIP was shown in . However, EAT was not found to be correlated with AIP in healthy subjects (r = 0.19, p = 0.21) ().
Correlation analysis between AIP and other variables
AIP was found to be positively correlated with BMI, serum albumin and total cholesterol as well as EAT ().
Table 3. Univariate correlation analysis of parameters of ESRD patients related to AIP.
Predictors of EAT
The stepwise linear regression analysis revealed that age, as well as BMI were independent predictors of EAT. Regression results are shown in .
Table 4. The independent predictors of EAT in linear regression analysis.
Discussion
There were five main findings of this study. First, AIP and EAT measurements were increased in ESRD patients when compared with healthy subjects. Second, EAT was significantly correlated with BMI and AIP in ESRD patients. Third, there was a statistically significant relationship between AIP and BMI, serum albumin and total cholesterol in ESRD patients. Fourth, AIP was significantly increased in ESRD patients who have higher EAT volumes. Lastly, age as well as BMI was found to be independent predictor of EAT.
Despite recent developments in the management of ESRD patients, CVD remains the main cause of morbidity and mortality in this population.Citation20,Citation21 This heightened risk can be attributed to many factors including advanced age, atherosclerosis, hypertension, dyslipidemia, anemia, hyperparathyroidism, chronic inflammation, diabetes (and its macrovascular and microvascular complications), left-ventricular hypertrophy, malnutrition and vascular calcification. Disturbed lipid profile was found to be closely related to atherosclerosis in general populationCitation22,Citation23 and in CKD patients.Citation4 Hemodialysis patients usually display increased concentrations of triglyceride-rich lipoproteins including very low density lipoprotein (VLDL), reduced serum levels of HDL-cholesterol and elevated concentrations of lipoprotein a (Lp(a)). Total and LDL-cholesterol values are usually within normal limits or slightly reduced in this population.Citation24
Despite the neutral effect of dialysis on serum lipid profile, certain dialysis-related parameters may significantly affect lipoprotein metabolism and modify the features of dyslipidemia in HD patients. It has also been demonstrated that the use of high-flux membranes is closely associated with a significant reduction in serum triglyceride levels as well as by an increase in HDL-cholesterol levels.Citation25,Citation26 This improvement might be attributed to an increase in the apolipoprotein C-II/C-III ratio which increases the activity of lipoprotein lipase and facilitates the intravascular lipolysis of triglyceride-rich lipoproteins and the type of dialysate that may significantly affect the serum levels of lipoproteins in this population.Citation4 Additionally, chronic heparin usage during HD sessions might be another important factor in which heparin releases lipoprotein lipase from the endothelial surface and this may result in defective catabolism of triglyceride-rich lipoproteins via decreasing the serum levels of lipoprotein lipase.Citation4 Lastly, the new non-calcium-containing phosphate binder sevelamer hydrochloride was found to be effective in decreasing total cholesterol and apolipoprotein B in HD patients.Citation27
Recent studies indicated that PD patients have higher serum triglyceride, Lp (a), small dense LDL-cholesterol levels and reduced HDL-cholesterol concentrations when compared to HD patients.Citation28,Citation29 This heightened risk might be attributed to many factors including lose of proteins via peritoneal membrane, increased absorption of glucose from dialysate and increased serum levels of insulin in this population.Citation30 In accordance to these study results, usage of icodextrin-containing dialysis solutions was found to be superior to glucose-containing dialysis solutions in terms of lowering serum triglycerides and small dense LDL concentrations.Citation31
EAT, like abdominal visceral adipose tissue, is a metabolically active tissue that can secrete proinflammatory cytokines and utilize free fatty acids (FFAs).Citation9 Despite the smaller adipocyte size, EAT has a higher rate of uptake and secretion of fatty acids than other visceral fat depots. In health, EAT may act as a “buffering system” by scavenging excess FFAs that are toxic to the myocardium. However, under ischemic conditions, EAT may serve as a local energy source by providing FFAs for the increased metabolism of the myocardium.Citation32,Citation33 In a recent study, the authors concluded that EAT acts as an extremely active organ that produces several bioactive adipokines, as well as proinflammatory and proatherogenic cytokines.Citation9–13 Levels of most of these proinflammatory cytokines were, in general, increased and these cytokines were found to be associated with atherosclerosis in ESRD patients.Citation34–36
In this study, we demonstrated that AIP and EAT measurements were increased in ESRD patients when compared with healthy subjects. Increased EAT was found to be positively correlated with advanced age, BMI and AIP in ESRD patients. AIP was significantly increased in ESRD patients who have higher EAT volumes. This association might be attributed to increased levels of proinflammatory cytokines secreted by EAT. Studies have shown an important association between obesity and cardiovascular morbidity and mortality in CKD populations.Citation20,Citation37,Citation38 Our findings were consistent with those of other studies. Therefore, defining EAT and AIP could be valuable for further studies such as the determination of cardiovascular risk stratification in ESRD patients.
Our study had two main limitations. First, this was a cross-sectional analysis of ESRD patients focusing on AIP and EAT. Second, the sample size was relatively small. This was not a prospective controlled study, so we cannot draw cause-and-effect relationships from our findings.
In conclusion, we found a relationship between EAT as defined by MDCT and AIP in ESRD patients. Further, clinical and experimental studies are needed to determine the relationship between EAT and AIP.
Declaration of interest
This study was supported by Scientific Investigation and Project Foundation of Selcuk University Meram School of Medicine.
References
- Magnus P, Beaglehole R. The real contribution of the major risk factors to the coronary epidemics: time to end the “only-50%” myth. Arch Intern Med. 2001;161(22):2657–2660
- Sarnak MJ, Coronado BE, Greene T, et al. Cardiovascular disease risk factors in chronic renal insufficiency. Clin Nephrol. 2002;57(5):327–335
- Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370(9602):1829–1839
- Tsimihodimos V, Dounousi E, Siamopoulos KC. Dyslipidemia in chronic kidney disease: an approach to pathogenesis and treatment. Am J Nephrol. 2008;28(6):958–973
- Sechi LA, Catena C, Zingaro L, Melis A, De Marchi S. Abnormalities of glucose metabolism in patients with early renal failure. Diabetes. 2002;51(4):1226–1232
- Despres JP, Lemieux I, Dagenais GR, Cantin B, Lamarche B. HDL-cholesterol as a marker of coronary heart disease risk: the Quebec cardiovascular study. Atherosclerosis. 2000;153(2):263–272
- Attman PO, Samuelsson O, Alaupovic P. Lipoprotein metabolism and renal failure. Am J Kidney Dis. 1993;21(6):573–592
- Ho E, Shimada Y. Formation of the epicardium studied with the scanning electron microscope. Dev Biol. 1978;66(2):579–585
- Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–2466
- Baker AR, Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1
- Kremen J, Dolinkova M, Krajickova J, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91(11):4620–4627
- Cheng KH, Chu CS, Lee KT, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond). 2008;32(2):268–274
- Fain JN, Sacks HS, Buehrer B, et al. Identification of omentin mRNA in human epicardial adipose tissue: comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int J Obes (Lond). 2008;32(5):810–815
- Turkmen K, Ozbek O, Kayikcioglu H, et al. The relationship between epicardial adipose tissue and coronary artery calcification in peritoneal dialysis patients. Cardiorenal Med. 2012;2(1):43–51
- Tonbul HZ, Turkmen K, Kayikcioglu H, Ozbek O, Kayrak M, Biyik Z. Epicardial adipose tissue and coronary artery calcification in diabetic and nondiabetic end-stage renal disease patients. Ren Fail. 2011;33(8):770–775
- Turkmen K, Kayikcioglu H, Ozbek O, et al. The relationship between epicardial adipose tissue and malnutrition, inflammation, atherosclerosis/calcification syndrome in ESRD patients. Clin J Am Soc Nephrol. 2011;6(8):1920–1925
- Frohlich J, Dobiasova M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem. 2003;49(11):1873–1880
- Dobiasova M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. 2001;34(7):583–588
- Schlett CL, Massaro JM, Lehman SJ, et al. Novel measurements of periaortic adipose tissue in comparison to anthropometric measures of obesity, and abdominal adipose tissue. Int J Obes (Lond). 2009;33(2):226–232
- Collins AJ. Cardiovascular mortality in end-stage renal disease. Am J Med Sci. 2003;325(4):163–167
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–S119
- Dobiasova M, Stribrna J, Sparks DL, Pritchard PH, Frohlich JJ. Cholesterol esterification rates in very low density lipoprotein- and low density lipoprotein-depleted plasma. Relation to high density lipoprotein subspecies, sex, hyperlipidemia, and coronary artery disease. Arterioscler Thromb. 1991;11(1):64–70
- Stampfer MJ, Krauss RM, Ma J, et al. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276(11):882–888
- Deighan CJ, Caslake MJ, McConnell M, Boulton-Jones JM, Packard CJ. Atherogenic lipoprotein phenotype in end-stage renal failure: origin and extent of small dense low-density lipoprotein formation. Am J Kidney Dis. 2000;35(5):852–862
- Blankestijn PJ, Vos PF, Rabelink TJ, van Rijn HJ, Jansen H, Koomans HA. High-flux dialysis membranes improve lipid profile in chronic hemodialysis patients. J Am Soc Nephrol. 1995;5(9):1703–1708
- Docci D, Capponcini C, Mengozzi S, Baldrati L, Neri L, Feletti C. Effects of different dialysis membranes on lipid and lipoprotein serum profiles in hemodialysis patients. Nephron. 1995;69(3):323–326
- Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62(1):245–252
- Kronenberg F, Lingenhel A, Neyer U, et al. Prevalence of dyslipidemic risk factors in hemodialysis and CAPD patients. Kidney Int Suppl. 2003;(84):S113–S116
- Siamopoulos KC, Elisaf M. Is CAPD atherogenic? Perit Dial Int. 1997;17(3):227–231
- Heimburger O, Stenvinkel P, Berglund L, Tranoeus A, Lindholm B. Increased plasma lipoprotein(a) in continuous ambulatory peritoneal dialysis is related to peritoneal transport of proteins and glucose. Nephron. 1996;72(2):135–144
- Babazono T, Nakamoto H, Kasai K, et al. Effects of icodextrin on glycemic and lipid profiles in diabetic patients undergoing peritoneal dialysis. Am J Nephrol. 2007;27(4):409–415
- Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes. 1990;14(12):1013–1022
- Wang TD LW, Chen MF. Epicardial adipose tissue measured by multidetector computed tomography: practical tips and clinical implications. Acta Cardiol. 2010;26:55–68
- Stenvinkel P, Heimburger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55(5):1899–1911
- Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102(21):2636–2642
- Yildiz A, Tepe S, Oflaz H, et al. Carotid atherosclerosis is a predictor of coronary calcification in chronic hemodialysis patients. Nephrol Dial Transplant. 2004;19(4):885–891
- Wang AY, Woo J, Lam CW, et al. Associations of serum fetuin-A with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol Dial Transplant. 2005;20(8):1676–1685
- Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray D, Barre PE. Outcome and risk factors of ischemic heart disease in chronic uremia. Kidney Int. 1996;49(5):1428–1434