Abstract
Coenzyme Q10 is a natural antioxidant and scavenger of free radicals. In the present study, we examined the effect of coenzyme Q10 on paraoxonase 1 (PON1) activity, lipid profile, atherogenic indexes and relationship of PON 1 activity by high-density lipoprotein (HDL) and atherogenic indexes in gentamicin (GM)-induced nephrotoxicity rats. Thirty Sprague–Dawley rats were divided into three groups to receive saline; GM, 100 mg/kg/d; and GM plus coenzyme Q10 by 15 mg/kg i.p daily, respectively. After 12 days, animals were anaesthetized, blood samples were also collected before killing to measure the levels of triglyceride (TG), cholesterol (C), low-density lipoprotein (LDL), very low density lipoprotein (VLDL), HDL, atherogenic indexes and the activities of PON1 of all groups were analyzed. Data were analyzed by non-parametric Mann–Whitney test (using SPSS 13 software). Coenzyme Q10 significantly decreased TG, C, LDL, VLDL, atherogenic index, atherogenic coefficient and cardiac risk ratio. HDL level and PON1 activity were significantly increased when treated with coenzyme Q10. Also, the activity of PON 1 correlated positively with HDL and negatively with atherogenic coefficient, cardiac risk ratio 1 and cardiac risk ratio 2. This study showed that coenzyme Q10 exerts beneficial effects on PON1 activity, lipid profile, atherogenic index and correlation of PON 1 activity with HDL and atherogenic index in GM -induced nephrotoxicity rats.
Introduction
Acute renal failure, characterized by decline in glomerular filtration rate, is the most manifestation of nephrotoxic damage. Researchers showed the incidence of renal dysfunction following aminoglycoside administration. Gentamicin (GM) is a commonly used aminoglycoside antibiotic that is effective against most of the Gram-negative microorganisms.Citation1 It has high antibacterial efficacy, rapid onset of action, low rate of true resistance, synergy with β-lactam antibiotics, and low cost; however, therapeutic doses of GM can cause nephrotoxicity, and it is among the most common causes of acute kidney injury.Citation2 Nephrotoxicity induced by GM is a complex phenomenon characterized by an increase in plasma lipid peroxidation and decrease catalase, glutathionepeoxidase and glutathione.Citation3 The toxicity of GM is believed to be related to the generation of reactive oxygen species (ROS) in the kidney.Citation4 The exact mechanism of GM-induced nephrotoxicity is unknown. However, GM has been shown to enhance the generation of ROS causing deficiency in intrinsic antioxidant enzymes.Citation5 ROS have been suggested as a cause of death for many cells in different pathological states including various models of liver, renal and cardiac diseases.Citation6
Also, clinical and experimental studies have shown that disturbing oxidant–antioxidant balance system is involved in the pathogenesis of chronic diseases such as cancer,Citation7 coronary heart disease, diabetes, liver injury and kidney injury.Citation8–10
Antioxidant and antioxidative enzyme activities reduce due to GM or increase of lipid peroxidation products.Citation11 Most research studies against GM -induced nephrotoxicity are focused on the use of various anti-oxidants.Citation12 A number of natural antioxidant such as vitamin E and phenolic compounds are known to have protective effects on liver injury and nephrotoxicity.Citation13 Chemical drugs such as antioxidant have many side effects; therefore, screening for new natural antioxidants is still attractive because they are safe and good alternative for the prevention of nephrotoxicity induced by GM. A growing body of research indicates that nutritional deficiencies such as antioxidants contribute to the development of nephrotoxicity. Coenzyme Q10 is a natural human ubiquinone, and has a fundamental role in mitochondrial energy (ATP) production in the respiratory chain.Citation14,Citation15 Coenzyme Q10 is also an antioxidant, scavenging free radicals and inhibiting lipid peroxidation.Citation16,The antioxidant effect of coenzyme Q10 is greater than vitamin E.Citation17 Coenzyme Q10 is also known to enhance the availability of other antioxidants such as vitamin C, vitamin E and beta-carotene.Citation18 Also, coenzyme Q10 inhibits low-density lipoproteins (LDL) oxidation in vitro and lipid peroxidation in vivo and scavenges free radicals. Also, several studies have shown that paraoxonase 1 (PON1) protects LDL and high density lipoprotein (HDL) against oxidative modification. PON1 as an antioxidant enzyme plays a key role in protecting LDL and HDL from oxidation by hydrolyzing activated phospholipids and lipid peroxide products.Citation19 Through these activities, serum PON1 plays an important role in the prevention of atherosclerosis.Citation20
Since the effects of coenzyme Q10 on PON1 activity, lipid profile, atherogenic index and liver enzymes activity in nephrotoxicity induced by GM in rats have not previously been reported; the objectives of the present study were to investigate effects of coenzyme Q10 on paraoxonase activity, lipid profile, atherogenic index and correlation of PON 1 activity with HDL and atherogenic indexes in nephrotoxicity induced by GM.
Materials and methods
Experimental design
Thirty male Sprague–Dawley rats (180–200 g) were prepared from Pasteur Institute of Tehran, and they were allowed to adapt themselves with the new location for 1 week. They were kept at a room temperature of 22 °C and a humidity of 50 ± 10% with 12-h light/dark cycles. This study was approved by the Animal Ethics Committee of Lorestan University of Medical Sciences and was in accordance with the National Health and Medical Research Council guidelines.
The animals were divided into three equal groups randomly including 10 rats each as follows: group 1 (control group), intraperitoneal saline injection, 0.25 mL/d; group 2, GM injection; group 3, GM and intraperitoneal coenzyme Q10, 15 mg/kg/d injection.Citation21 GM, 100 mg/kg/d, was injected intraperitoneally for 12 days, 1 h before GM injection.Citation22 After the last injection of GM, all the animals were immediately kept in individual, metabolic cages in order to collect 24-h urine. Blood samples were obtained from animals’ hearts under anesthesia (nesdonal, 50 mg/kg, intraperitoneal), were allowed to clot for 20 min in laboratory temperature, and were centrifuged at 3000 rpm for 15 min for serum separation. Then, the kidneys and livers were excised and used for homogenization. The kidneys and livers were homogenized in Tris–HCl buffer (0.05 mol/L Tris–HCl and 1.15% KCl, pH 7.4), using a homogenizer. The homogenate was centrifuged at 18,000 × g (4 °C) for 30 min. The supernatant was utilized for kidneys and livers tissue biochemical analysis.
Determination of lipid profile and atherogenic index
The serum levels of fasting blood sugar, triglyceride (TG), cholesterol (C), LDL, very low-density lipoprotein (VLDL), HDL, and atherogenic index of all groups were analyzed. Cholesterol and triglyceride concentrations were measured by biochemical analyzer using commercial kits (Olympus AU-600, Tokyo, Japan). HDL was measured in the supernatant after the precipitation of the Apo-B containing lipoproteins (LDL and VLDL) using polyanions in the presence of a divalent cation.Citation23 LDL and VLDL were determined by calculation using the Freidewald et al. equation.Citation24 The atherogenic index was determined by calculation using the Ikewuchi and Ikewuchi equation.Citation25
Determination of lipid peroxidation
Serum levels of malondialdehyde (MDA), as a product of lipid peroxidation, were measured by the thiobarbituric acid (TBA) assay. Absorbances were measured spectrophotometrically at 532 nm and the concentrations were expressed as nmol MDA/mg-pr.Citation26
Determination o f PON1 activity
PON activity was determined using paraoxon as a substrate and measured by increases in the absorbance at 412 nm due to the formation of 4-nitrophenol as already described.Citation27 The activity was measured at 25 °C by adding 50 μL of serum to 1 mL Tris–HCl buffer (100 mM at pH 8.0) containing 2 mM CaCl2 and 5 mM of paraoxon. The rate of generation of 4-nitrophenol was determined at 412 nm. Enzymatic activity was calculated using molar extinction coefficient 17,100 M−1 cm−1.
Statistical analysis
All values are expressed as mean ± SE. The data were compared between groups by Mann–Whitney U test. Statistical analyses were performed using the SPSS 13 (Chicago, IL) for windows software. A p value of <0.05 was considered statistically significant.
Results
Lipid profile and atherogenic indexes
The level of fasting blood glucose (FBG) in the untreated nephrotoxic rats was significantly (1.29-fold) higher than that of control animals. The treatment of nephrotoxic animal with coenzyme Q10 could significantly (24.83%) inhibit the increase of FBG in comparison with the untreated nephrotoxic animals (). The level of cholesterol in the untreated nephrotoxic rats was (1.17-fold) higher than that of control animals. The treatment of nephrotoxic animal with coenzyme Q10 could not inhibit the increase of cholesterol in comparison with the untreated nephrotoxic animals (). The level of TG in the untreated nephrotoxic rats was significantly (1.47-fold) higher than that of control animals. The treatment of nephrotoxic animal with coenzyme Q10 could significantly (43.77%) inhibit the increase of TG. in comparison with the untreated nephrotoxic animals ().
Table 1. Effect of coenzyme Q10 on TC, TG, LDL, HDL and VLDL and in nephrotoxic rats.
The level of LDL in the untreated significantly (4.72-fold) nephrotoxic rats were higher than that of control animals. The treatment of nephrotoxic animal with coenzyme Q10 could not inhibit the increase of LDL in comparison with the untreated nephrotoxic animals (). The level of VLDL in the untreated nephrotoxic rats was significantly (1.47-fold) higher than that of control animals. The treatment of nephrotoxic animal with coenzyme Q10 could significantly inhibit significantly the increase of VLDL in comparison with the untreated nephrotoxic animals ().
The level of HDL in the untreated nephrotoxic rats was significantly (0.41-fold) lower than that of control animals. The treatment of nephrotoxic animal with coenzyme Q10 could of HDL in comparison with the untreated nephrotoxic animals ().
The level of atherogenic index (units) (log (TG/HDLC)) in the untreated nephrotoxic rats was significantly (1.46-fold) higher than that of control animals. The treatment of nephrotoxic animal with coenzyme Q10 could significantly (43.72%) inhibit the increase of atherogenic index in comparison with the untreated nephrotoxic animals (). The level of Atherogenic Coefficient ((TC-HDLC)/HDLC) in the untreated nephrotoxic rats was significantly (2.09-fold) higher than that of control animals. The treatment of nephrotoxic animal with coenzyme Q10 could significantly (47.66%) inhibit the increase of Atherogenic Coefficient. in comparison with the untreated nephrotoxic animals (). The level of Cardiac Risk Ratio (TC/HDL-C) in the untreated nephrotoxic rats was significantly (5.86-fold) higher than that of control animals. The treatment of nephrotoxic animal with coenzyme Q10could significantly (47.87%) inhibit the increase of Cardiac Risk Ratio (TC/HDL-C) in comparison with the untreated nephrotoxic animals (). The level of Cardiac Risk Ratio (LDL/HDL-C) in the untreated nephrotoxic rats was significantly (3.22-fold) higher than that of control animals. The treatment of nephrotoxic animal with coenzyme Q10 could significantly (39.96%) inhibit the increase of Cardiac Risk Ratio (LDL/HDL-C) in comparison with the untreated nephrotoxic animals ().
Table 2. Effect of coenzyme Q10 on atherogenic index, atherogenic coefficient, and cardiac risk ratio in nephrotoxic rats.
The activity of PON 1 in the untreated nephrotoxic rats was significantly (1.59-fold) higher than that of control animals. The treatment of nephrotoxic animal with oleuropein could not inhibit the increase of activity of significantly (31.33%) higher than in comparison with the untreated nephrotoxic animals ().
Table 3. Effect of coenzyme Q10 on serum lipid peroxidation and PON 1 activity in nephrotoxic rats.
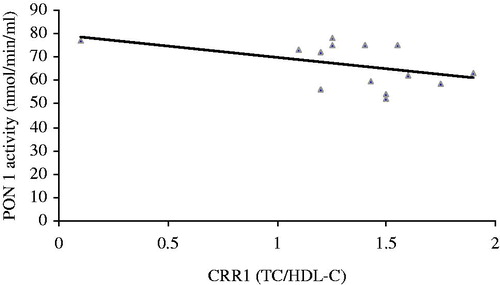
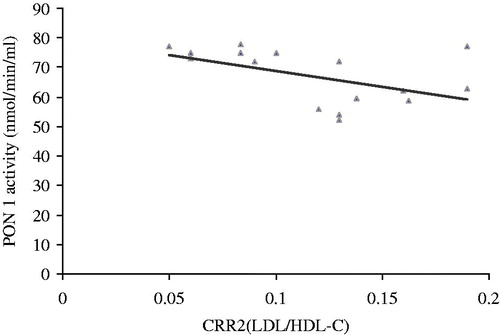
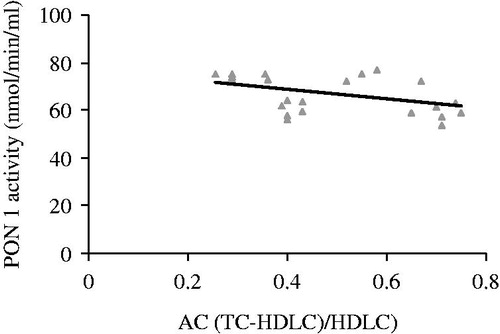
The activity of PON 1 correlated positively with HDL (r = −0.755, p = 0.000, ). The activity of PON 1 correlated negatively with atherogenic coefficient (r = −0.185, p = 0.035, ), cardiac risk ratio 1 (r = −0.236, p = 0.016, ) and cardiac risk ratio 2 (r = −0.314, p = 0.004, ).
Figure 1. Correlation between maternal serum PON 1 activity and levels of HDL in nephrotoxic rats treated with coenzyme Q10 (r = −0.755, p = 0.000).
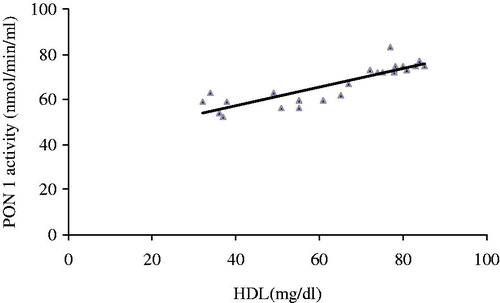
Figure 2. Correlation between maternal serum PON 1 activity and levels of AC (atherogenic coefficient (TC-HDLC/HDLC)) in nephrotoxic rats treated with coenzyme Q10 (r = −0.185, p = 0.035).
Discussion
Effect of coenzyme Q10 on serum lipid profile, atherogenic index and lipid peroxidation
Nephrotoxicity significantly increased serum FBG, TG, Cholesterol, VLDL and LDL concentrations in comparison with the control group. Treatment of nephrotoxic animals with coenzyme Q10 significantly inhibited increase of serum FBG, TG, Cholesterol, VLDL and LDL concentrations, atherogenic index, atherogenic coefficient and cardiac risk ratio in comparison with the untreated nephrotoxic animals. Also, treatment of nephrotoxic animals with coenzyme Q10 significantly inhibited decrease of serum HDL concentrations in comparison with the untreated nephrotoxic animals. In our study, treatment of nephrotoxic animals with coenzyme Q10 slightly inhibited increase of serum MDA concentrations in comparison with the untreated nephrotoxic animals, but was not significant. There are reports that natural antioxidant such as quercetin and vitamin C has hypolipidemic effects.Citation28,Citation29 Also, there are reports that coenzyme Q10 have hypolipidemic effects. Cicero et al. showed coenzyme Q10 could reduce serum lipoprotein (a) level in patients with high serum triglyceride levels. Moreover, Singh et al. showed coenzyme Q10 could reduce serum lipoprotein (a) level in patients with coronary artery diseases. Also, Shojaei et al. showed coenzyme Q10 could reduce serum levels of lipoprotein (a) and lipids in maintenance hemodialysis patients on statin therapy.Citation30–32 Results of our study are in accordance with others researchers’ study that showed coenzyme Q10 could reduce serum lipid and lipoprotein level. Therefore, natural antioxidant with hypolipidemic effects could prevent or be helpful in reducing the complications of lipid profile seen in nephrotoxic patients. The mechanisms by which coenzyme Q10 can decrease high serum lipid level is not well known. The mechanism of hypolipidemic and antiatherogenic action of natural antioxidant may be due to the inhibition of dietary lipid absorption in the intestine or its production by liver or stimulation of the biliary secretion of cholesterol and cholesterol excretion in the faces.Citation33,Citation34
Moreover, coenzyme Q10 is a lipid-soluble molecule, and it is present in sufficient amounts in lipoprotein (a). Supplementation with coenzyme Q10 can inhibit expression of lipoprotein (a) receptor and result in decreased serum lipoprotein (a).Citation35 Also, the mechanism of hypolipidemic and antiatherogenic action of natural antioxidant may be due to the inhibition of glycation lipoproteins, enzymes and proteins that involve lipid and lipoprotein metabolism.Citation36
Effect of coenzyme Q10 on serum PON 1 activity and correlation of PON 1 activity with HDL and atherogenic index
Serum PON 1 activity significantly was decreased in the untreated nephrotoxic animals in comparison with the control group. Treatment of the nephrotoxic animals with coenzyme Q10 could significantly elevate decreasing of serum PON 1 activity in comparison with the untreated nephrotoxic animals. The most relevant finding of this study is that activity of PON 1 correlated positively with HDL and negatively with atherogenic coefficient, cardiac risk ratio 1 and cardiac risk ratio 2 in treated nephrotoxic animals.
Researchers showed that PON1 as an antioxidant enzyme inhibit oxidative modification of LDL and contribute to most of the antioxidative activity that has been attributed to HDL.Citation37 PON1 activity was positively correlated with HDL-C levels.Citation38 In contrast, PON1 activity was inversely correlated with atherogenic index; thus, it can be suggested that decreased PON1 activity may be, in part, due to consumption of PON1 for the prevention of oxidation.Citation39,Citation40 It strongly suggests that decreased PON1 activity may be at least partially related to the consumption of PON1 caused by oxidative stress process.Citation41 Paraoxonase (PON1) is an ester hydrolase that has both arylesterase and paraoxonase activities.Citation42 Recently, roles for PON1 in a number of processes have been studied, including lipid and lipoprotein metabolism, as well as for their antiatherogenic and antioxidant properties.Citation43
Results of our study are in accordance with others researchers’ study that showed coenzyme Q10 could increase PON 1 activity and increase of PON 1 activity has positive correlation with HDL and positive correlation with atherogenic index. Also, researchers indicated that coenzyme Q10 is found to possess a good antioxidant activity.Citation30 Also, researchers reported the role of oxidative stress as a central factor in onset and progression of nephrotoxic complications such as hyperlipemia and hepatic damage.Citation44 In spite of numerous reports, our results showed efficacy of antioxidative supplements administration in the prevention of nephrotoxic complications. Because antioxidant therapy is one of the most important treatment strategies for nephrotoxic patients for the prevention and slowing of nephrotoxic complications progression such as hyperlipemia, hepatic damage.
Conclusion
This study showed that coenzyme Q10 has beneficial effects including; (1) reducing the elevated serum lipid peroxidation, (2) reducing the elevated serum lipid profile and atherogenic index, (3) increasing the reduced serum PON 1 activity, (4) increasing the reduced serum HDL level of nephrotoxic rats. Also, this study showed that PON 1 activity was correlated positively with HDL and negatively with atherogenic index. Hence, attenuation of PON 1 activity, liver enzyme markers lipid profile and atherogenic index can decrease the risk of cardiovascular death in nephrotoxic patients.
Declaration of interest
The authors declare no conflicts of interests. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors thank the head and personnel of Razi Herbal Drugs Research Center of Lorestan Medical University.
References
- Nasri H, Nematbakhsh M, Ghobadi S, Ansari R, Shahinfard N, Rafieian-Kopaei M. Preventive and curative effects of ginger extract against histopathologic changes of gentamicin-induced tubular toxicity in rats. Int J Prev Med. 2013;4(3):316–321
- McKeage K, Deeks ED. Doxycycline 40 mg capsules (30 mg immediate-release/10 mg delayed-release beads): anti-inflammatory dose in rosacea. Am J Clin Dermatol. 2010;11(3):217–222
- Manikandan R, Beulaja M, Thiagarajan R, Priyadarsini A, Saravanan R, Arumugam M. Ameliorative effects of curcumin against renal injuries mediated by inducible nitric oxide synthase and nuclear factor kappa B during gentamicin-induced toxicity in Wistar rats. Eur J Pharmacol. 2011;670(2–3):578–585
- Banday AA, Farooq N, Priyamvada S, Yusufi AN, Khan F. Time dependent effects of gentamicin on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in rat kidney tissues. Life Sci. 2008;82(9–10):450–459
- Cristóvão C, Cristóvão L, Nogueira F, Bicho M. Evaluation of the oxidant and antioxidant balance in the pathogenesis of chronic obstructive pulmonary disease. Rev Port Pneumol. 2012;19(2):70–75
- Whaley-Connell A, Sowers JR. Oxidative stress in the cardiorenal metabolic syndrome. Curr Hypertens Rep. 2012;14(4):360–365
- Razavi A, Baghshani MR, Rahsepar AA, et al. Association between C-reactive protein, pro-oxidant-antioxidant balance and traditional cardiovascular risk factors in an Iranian population. Ann Clin Biochem. 2013;50(2):115–121
- Kaya Y, Çebı A, Söylemez N, Demır H, Alp HH, Bakan E. Correlations between oxidative DNA damage, oxidative stress and coenzyme Q10 in patients with coronary artery disease. Int J Med Sci. 2012;9(8):621–626
- Piwkowska A, Rogacka D, Audzeyenka I, Jankowski M, Angielski S. High glucose concentration affects the oxidant antioxidant balance in cultured mouse podocytes. J Cell Biochem. 2011;112(6):1661–1672
- Orellana M, Rodrigo R, Thielemann L, Guajardo V. Bile duct ligation and oxidative stress in the rat: effects in liver and kidney. Comp Biochem Physiol C Toxicol Pharmacol. 2000;126(2):105–111
- Taye A, Ibrahim BM. Activation of renal heme oxygenase alleviates gentamicin-induced acute nephrotoxicity in rats. J Pharm Pharmacol. 2013;65(7):995–1004
- Randjelovic P, Veljkovic S, Stojiljkovic N, et al. Protective effect of selenium on gentamicin-induced oxidative stress and nephrotoxicity in rats. Drug Chem Toxicol. 2012;35(2):141–148
- Nematbakhsh M, Ashrafi F, Safari T, et al. Administration of vitamin E and losartan as prophylaxes in cisplatinM induced nephrotoxicity model in rats. J Nephrol. 2012;25(3):410–417
- Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol. 2007;37(1):31–37
- Somayajulu M, McCarthy S, Hung M, Sikorska M, Borowy-Borowski H, Pandey S. Role of mitochondria in neuronal cell death induced by oxidative stress; neuroprotection by coenzyme Q10. Neurobiol Dis. 2005;18(3):618–627
- Bélanger MC, Mirault ME, Dewailly E, Berthiaume L, Julien P. Environmental contaminants and redox status of coenzyme Q10 and vitamin E in Inuit from Nunavik. Metabolism. 2008;57(7):927–933
- Mabuchi H, Higashikata T, Kawashiri M, et al. Reduction of serum ubiquinol-10 and ubiquinone-10 levels by atorvastatin in hypercholesterolemic patients. J Atheroscler Thromb. 2005;12:111–119
- Shekelle P, Morton S, Hardy ML. Effect of supplemental antioxidants vitamin C, vitamin E, and coenzyme Q10 for the prevention and treatment of cardiovascular disease. Evid Rep Technol Assess. 2003;83:1–3
- Toy H, Camuzcuoglu H, Celik H, Erel O, Aksoy N. Assessment of serum paraoxonase and arylesterase activities in early pregnancy failure. Swiss Med Wkly. 2009;139(5–6):76–81
- Mackness M, Mackness B. Targeting paraoxonase-1 in atherosclerosis. Expert Opin Ther Targets. 2013;17(7):829–837
- Ahmadvand H. Effects of coenzyme Q10 on hemoglobin A1C, serum urea and creatinine in alloxan-induced type 1 diabetic rats. IJPT. 2012;11:64–67
- Tavafi M, Ahmadvand H, Toolabi P. Inhibitory effect of olive leaf extract on gentamicin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2012;6(1):25–32
- Warnick GR, Cheung MC, Albers JJ. Comparison of current methods for HDL quantification. Clin Chem. 1979;25:596–601
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of LDL-C in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502
- Ikewuchi JC, Ikewuchi JC. Alteration of plasma lipid profiles and atherogenic indeces by Stachytarpheta iamaicensis L. (Vahl). Biokemistri. 2009;21(2):71–77
- Ahmadvand H, Tavafi M, Khosrowbeygi A. Amelioration of altered antioxidant enzymes activity and glomerulosclerosis by coenzyme Q10 in alloxan-induced diabetic rats. J Diabetes Compl. 2012;26(6):476–482
- Azizi F, Rahmani M, Raiszadeh F, Solati M, Navab M. Association of lipids, lipoproteins, apolipoproteins and paraoxonase enzyme activitywith premature coronary artery disease. Coron Artery Dis. 2002;13(1):9–16
- Askari G, Hajishafiee M, Ghiasvand R, et al. (2013) Quercetin and vitamin C supplementation: effects on lipid profile and muscle damage in male athletes. Int J Prev Med. 2013;1:S58–S62
- Milton Prabu S, Muthumani M, Shagirtha K. Quercetin potentially attenuates cadmium induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. Eur Rev Med Pharmacol Sci. 2013;17(5):582–595
- Cicero AF, Derosa G, Miconi A, Laghi L, Nascetti S, Gaddi A. Treatment of massive hypertriglyceridemia resistant to PUFA and fibrates: a possible role for the coenzyme Q10? Biofactors. 2005;23(1):7–14
- Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L. Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221(2):311–316
- Shojaei M, Djalali M, Khatami M, Siassi F, Eshraghian M. Effects of carnitine and coenzyme Q10 on lipid profile and serum levels of lipoprotein(a) in maintenance hemodialysis patients on statin therapy. Iran J Kidney Dis. 2011;5(2):114–118
- Garjani A, Fathiazad F, Zakheri A, et al. The effect of total extract of Securigera securidaca L. seeds on serum lipid profiles, antioxidant status, and vascular function in hypercholesterolemic rats. J Ethnopharmacol. 2009;126(3):525–532
- Jayasooriya AP, Sakono M, Yukizaki C, Kawano M, Yamamoto K, Fukuda N. Effects of Momordica charantia powder on serum glucose levels and various lipid parameters in rats fed with cholesterol-free and cholesterol-enriched diets. J Ethnopharmacol. 2000;72(1–2):331–336
- Mori M, Sawashita J, Kitano M, et al. Supplementation with the reduced form of Coenzyme Q10 decelerates phenotypic characteristics of senescence and induces a peroxisome proliferator-activated receptor-alpha gene expression signature in SAMP1 mice. Mol Nutr Food Res. 2010;54(6):805–815
- Harris CS, Beaulieu LP, Fraser MH, et al. Inhibition of advanced glycation end product formation by medicinal plant extracts correlates with phenolic metabolites and antioxidant activity. Planta Med. 2011;77(2):196–204
- Aviram M, Kaplan M, Rosenblat M, Fuhrman B. Dietary antioxidants and paraoxonases against LDL oxidation and atherosclerosis development. Handb Exp Pharmacol. 2005;(170):263–300
- Mohamadin AM, Habib FA, Elahi TF. Serum paraoxonase 1 activity and oxidant/antioxidant status in Saudi women with polycystic ovary syndrome. Pathophysiology. 2010;17(3):189–196
- Göçmen AY, Sahin E, Koçak H, Tuncer M, Gümüşlü S. Levels of asymmetric dimethylarginine, nitric oxide and lipid peroxidation markers in patients with end-stage renal disease having peritoneal dialysis treatment. Clin Biochem. 2008;41(10--11): 836–840
- Razavi AE, Ani M, Pourfarzam M, Naderi GA. Associations between high density lipoprotein mean particle size and serum paraoxonase-1 activity. J Res Med Sci. 2012;17(11):1020–1026
- Yilmaz N, Simsek N, Aydin O, et al. Decreased paraoxonase 1, arylesterase enzyme activity, and enhanced oxidative stress in patients with mitral and aortic valve insufficiency. Clin Lab. 2013;59(5--6):597–604
- Bulbuller N, Eren E, Ellidag HY, et al. Diagnostic value of thiols, paraoxonase 1, arylesterase and oxidative balance in colorectal cancer in human. Neoplasma. 2013;60(4):419–424
- Ferretti G, Bacchetti T, Masciangelo S, Grugni G, Bicchiega V. Altered inflammation, paraoxonase-1 activity and HDL physicochemical properties in obese humans with and without Prader--Willi syndrome. Dis Model Mech. 2012;5(5):698–705
- Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12(1):5–18