Abstract
Background: Acute kidney injury induced by aristolochic acid (AA) might occur in patients with chronic glomerular nephritis (CGN). In this study, the clinical and pathological features of patients with acute aristolochic acid nephropathy (AAN) superimposing CGN (AAN-CGN) were investigated. Methods: Eighteen patients diagnosed as acute AAN were included in this retrospective study, from January 2001 to December 2009. According to the pre-existing CGN, 13 patients were identified as the AAN-CGN group, and 5 isolated AAN patients as the control group. Clinical and pathological features were compared between the two groups. Results: In the AAN-CGN group, six patients complained with gastrointestinal symptoms, such as nausea, vomiting, or loss of appetite. The rest of seven cases were asymptomatic or minimally uncomfortable, who were found with elevated serum creatinine (Scr) in the follow up of CGN. Compared with the control group, the patients in AAN-CGN group had higher levels of serum uric acid, urine n-acetyl-β-d-glucosaminidase, and urine protein excretion (366.2 ± 122.8 vs. 218.0 ± 125.8 μmol/L, p = 0.037; 9.74 ± 4.4 vs. 1.38 ± 1.01 g/d, p = 0.001; 61.2 ± 21.9 vs. 27.4 ± 15.8 μ/g ċ cr, p = 0.007, respectively). In addition to, the AAN-CGN patients had an absolutely prominent percentage of macromolecule substance in the urine protein electrophoresis (25.0 ± 6.32 vs. 15.8 ± 7.8%, p = 0.029). The occurrence of hypokalemia and excretion of aminoaciduria were lower than that in the control group. Pathologically, 84.6% of patients were found with tubular brush border dropping, 30.8% with naked tubular basement membrane, and 15.4% with different stages of vascular lesion. There were no statistical differences in the above-mentioned pathological parameters between the two groups. In the follow-up, 10 patients with AAN-CGN recovered with normal Scr, accounting for 76.9%, which was better than the recovery in the control group. Conclusion: Patients with acute AAN-CGN manifested with a great mass of urine protein excretion, low incidence of hypokalemia and aminoaciduria, however, the tubular-interstitial lesions were similar to the isolated AAN.
Introduction
Aristolochic acid nephropathy (AAN) has been a recognized cause of progressive chronic kidney disease (CKD) worldwide for about 20 years.Citation1 The typical feature of AAN was described as extensive interstitial fibrosis and severe loss of renal tubules along with a rapid progression to end-stage renal disease. The 2-year renal survival rate has been reported to be only 17%,Citation2 which is much worse than the outcome for other tubulointerstitial nephropathies. And according to our own data,Citation3 the chronic AAN had unsatisfied prognosis which might be correlated with the gender, proteinuria, RBP, and glucosuria.
Though the Food and Drug Administration issued an alert in 2001 warning consumers and the herbal medicine industry of the dangers of AA, some patients still use these medications without a doctor’s prescription or guidance. AAN is still a worldwide problem, especially in Asian countries. Nephrologists started to collect medication histories from all of their CKD patients as routine clinical practice, especially from those presenting with chronic tubulointerstitial nephropathy of unknown cause. So there were lots of papers about the isolated AAN and many chronic AAN cases were recognized at last two decade. At the same time, we found that in the clinical practice, acute kidney injury induced by AA occasionally occurred in patients with primary CGN. The aim of this study is to explore the clinical and pathological characteristics of patients with acute AAN superimposing primary CGN (AAN-CGN).
Materials and methods
Patient selection
Patients who were diagnosed as acute AAN in our hospital from January 2001 to December 2009 were enrolled in this retrospective study.
Criteria for diagnosis
Acute AAN was diagnosed according to (1) serum creatinine rising by ≥26 µmol/L within 48 h or serum creatinine rising ≥1.5-fold from the reference value, which is known or presumed to have occurred within one week; (2) definite history of taking AA-containing medications prior to the disease onset; (3) the presence of obvious tubular dysfunction; (4) the absence of recent or long-term ingestion of antibiotics, nonsteroidal anti-inflammatory drugs, diuretics, or Chinese traditional medicines containing minerals; (5) no evidences of infection, hypovolemia, urine tract obstruction, or AKI induced by other autoimmunity diseases. There were 19 patients diagnosed with acute AAN from 2001 to 2009, including one case which was also confirmed as myeloma that might involve with both glomerular and tubular. And, the myeloma case was excluded. Eventually, 18 patients with acute AAN were included in this investigation, comprising 13 subjects of AAN-CGN (Group 1) and 5 isolated acute AAN (Group 2).
Clinical data
Urine and blood data were collected from medical records. The basic information about age, gender, and the main complaints at onset were recorded. Urine data included glucose, amino-acid, and protein quantification, sediment analysis, n-acetyl-β-d-glucosaminidase (NAG), retinol binding protein (RBP), protein electrophoresis, and osmolality. Blood data included hemoglobin, serum creatinine (Scr), uric acid, and serum potassium.
Histological data
Renal biopsy was performed in all patients. Biopsy tissue was studied under light microscope. Biopsy materials were fixed in 0.1 M phosphate-buffered 10% formalin. Paraffin sections were stained with hematoxylin and eosin, periodic acid-Schiff, Masson trichrome, and methenamine silver.
Statistical analysis
For descriptive statistical analysis, continuous variables were expressed as mean ± standard deviation. Categorical variables were stated as proportions (percentage). Binomial variables were compared between the 2 groups using χ2 or Fisher’s exact tests, and continuous variables were analyzed by Mann–Whitney U test. A p value of <0.05 was considered as a significant value. Results were reported as odds ratios with 95% confidence intervals. All data were analyzed with SPSS 13.0 software (SPSS, Chicago, IL).
Results
General conditions
In total, 18 subjects were diagnosed acute AAN, including 13 males and 5 females, with an average of 35.3 ± 14.6 years old (ranging from 20 to 65), shown in . In group one, they were 5 patients with IgA nephropathy, 4 membranous nephropathy, 3 podocyte diseases, and 1 minimal change disease. Gastrointestinal symptoms such as nausea, vomiting and poor appetite were seen in 10 patients, including 6 in group 1 and 4 in group 2. Three patients were found with hypertension. In group 1, there are seven patients presented with no obvious symptoms who were found with elevated Scr during the follow-up of the CGN and no patients presented with oliguria.
Table 1. General conditions of patients with AAN-CGN.
Laboratory data
As shown in , there was no difference found in the levels of Scr, urine retinol binding protein, and urine osmolality between the two groups. Incidences of anemia and glucosuria were similar in the two groups. Furthermore, patients in AAN-CGN group had higher levels of serum uric acid, urine n-acetyl-β-d-glucosaminidase, and urine protein excretion (366.2 ± 122.8 vs. 218.0 ± 125.8 μmol/L, p = 0.037; 9.74 ± 4.4 vs. 1.38 ± 1.01 g/d, p = 0.001; 61.2 ± 21.9 vs. 27.4 ± 15.8 μ/g ċ cr, p = 0.007, respectively). Compared with the group 2, the AAN-CGN patients had an absolutely prominent percentage of macromolecule substance in the urine protein electrophoresis (25.0 ± 6.32 vs. 15.8 ± 7.8%, p = 0.029). The occurrence of hypokalemia and excretion of aminoaciduria were lower than those in the group 2.
Table 2. Laboratory findings of patients with AAN-CGN.
Renal pathological features
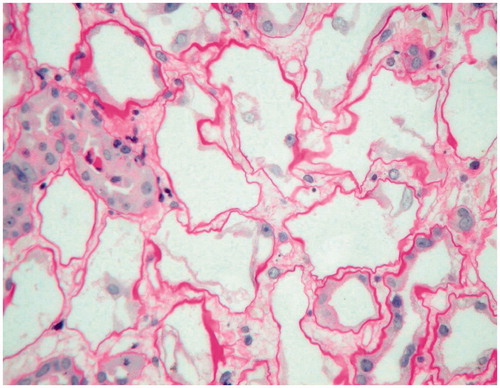
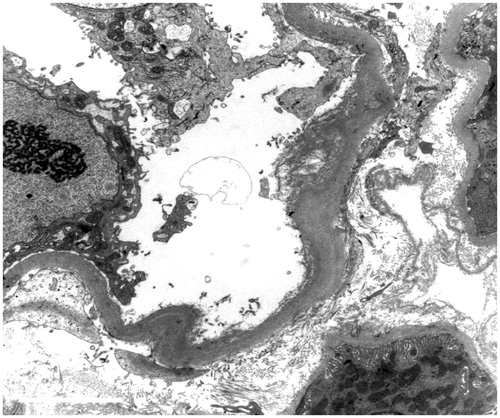
Percutaneous renal biopsies were performed in all patients under type B ultrasound guiding. Lesions of the tubular interstitial area were shown in . In group 1, 84.6% of patients were found with tubular brush border dropping, 30.8% with naked tubular basement membrane and 15.4% ( and ) with different stages of vascular lesion. The incidence of naked tubular basement membrane was higher in group 2. However, there were no statistical differences in the above-mentioned pathological parameters between the two groups.
Table 3. Pathological features of the tubular interstitium in patients with AAN-CGN.
Follow-up
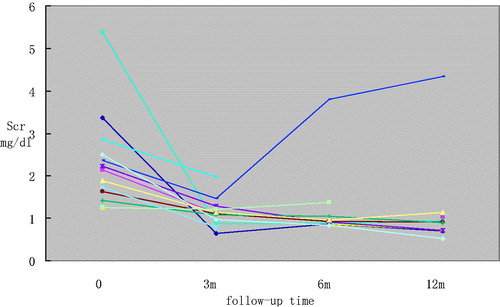
All patients were asked to avoid taking AA containing drugs again and were given Cordyceps sinensis to promote recovery of the tubular. The AAN-CGN patients were also treated with Triptergium wilfordii or prednisone for the original glomerular diseases. One year after hospital discharge, 11 patients got complete renal function recovery, including 10 subjects in group 1 and 1 subject in group 2. The rest of three cases in group 1 did not gain satisfying improvement. Follow-up of Scr in patients with AAN-CGN was presented in . One patient presented with advanced renal impairment, one with partial remission, and one with stable Scr level. The recovery rate was higher in group 1 than that in group 2 (76.9% vs. 20.0%, p = 0.047).
Discussion
Aristolochic acid nephropathy (AAN) was a recognized cause of progressive chronic kidney disease (CKD) worldwide,Citation1 and the incidence of this disease was lower more and more. Though the Food and Drug Administration issued an alert in 2001 warning consumers and the herbal medicine industry of the dangers of AA, some patients still use these medications without a doctor’s prescription or guidance. AAN is still a worldwide problem, especially in Asian countries. In the clinical practice, acute kidney injury induced by AA occasionally occurred in patients with primary CGN. In this article, we demonstrated that those patients had higher levels of serum uric acid, urine n-acetyl-β-d-glucosaminidase, and urine protein excretion. In addition to, the AAN-CGN patients had an absolutely prominent percentage of macromolecule substance in the urine protein electrophoresis, a low occurrence of hypokalemia and aminoaciduria, however, the tubular-interstitial lesions were similar to the isolated acute AAN.
Clinically, the most common complaint was gastrointestinal symptoms in this study, but most patients were found with elevated Scr during the follow up of the pre-existing CGN. For the AAN-CGN, the patients presented with great mass of urine protein excretion (more than 3.5 g/d), no matter what kind of pathological changes of glomerular was. It might be associated with the down regulation of megalin expression in proximal renal tubular by AA.Citation3 On the other hand, urine NAG was extremely elevated in AAN-CGN patients, which was a sensitive marker of the damage to renal tubular. However, excretion of urine protein itself might lead to the abnormal NAG. Patients with AAN were usually companied with hypokalemia, glucosuria, and aminoaciduria, which prompt proximal renal tubular damage. In our study, patients with AAN-CGN had lower occurrence of hypokalemia and less excretion of aminoaciduria, which meant less damage to the tubular cells. As we know, the toxic effects of AA were attributable to metabolic conversion to reactive electrophilic intermediates, which lead to a variety of deleterious effects. Recently, Priestap and his colleagues investigated the metabolism process of AA-I in the isolated perfusing rat kidney.Citation4 They found that AA-I distributed rapidly and extensively in kidney tissues by uptake from the peritubular capillaries and the tubules. And, the filtered AA-I was reabsorbed at the tubules, whereas its metabolites were secreted. Therefore, it may suppose that the renal tubular damage had already occurred due to the persistent protein excretion in our patients with AAN-CGN before they took in the AAs, which led to less uptake or reabsorption of AAs at the tubules.
In experiment model of acute AAN, the renal function and tubular lesions gradually recovered,Citation5 but tumors and preneoplastic proliferation might occur.Citation6 Few studies have been reported that the incidence of renal function recovery ranged from 0% to 15.4% in patients with acute AAN,Citation7,Citation8 which excluded cases with glomerular disease. Recently, three newborns with acute aristolochic acid nephropathy were reported by WangCitation9 and all of them gained normal renal function after treatment. And, later in Chen’s report of four adult patients with acute AAN,Citation10 two subjects got completely remission, while the other two slipped to chronic renal failure. The authors considered the prognosis of acute AAN related with age. Up to now, there was few data about the prognosis of AAN-CGN. Only a case report of a patient with FSGS who developed acute renal failure after a 10-day history of daily ingestion of a Chinese herbal medicine.Citation11 The patient was treated with prednisolone and hemodialysis. The renal function finally recovered. In our study, the recovery rate was higher for patients with AAN-CGN. It might be associated with that these patients received immunosuppressive agents, angiotensin converting enzyme inhibitor/angiotensin II receptor blockers or prednisone for the treatment of primary CGN. The treatment might decrease the toxicity of AA, attenuate the interstitial destruction and slow down the deterioration of renal function. Recently, it was reported that Cozaar, the widely used angiotensin II receptor blocker, could significantly ameliorate the renal microvascular injury and protect renal function in the experiment AAN model.Citation12 However, it was still ambiguous about the effect of the steroid therapy on AAN, which need to be investigated by more prospective random control clinical researches.Citation13,Citation14
In fact, due to the persistent or relapse of urine protein, many patients with CGN went for kinds of Chinese herbs which might contain aristolochic acids. However, not all individuals exposed to AA are susceptible to develop AAN. ChenCitation15 suggested that glutathione s-transferases T1 null genotype is associated with susceptibility to aristolochic acid nephropathy.
In summary, patients with acute AAN-CGN manifested with a great mass of urine protein excretion. The hypokalemia and the extent of aminoaciduria were less obvious. Furthermore, the tubular-interstitial lesions were similar to the isolated AAN. As a result, for acute kidney injury in patients with CGN, more attention should be paid to the medication history, especially for the AA containing drugs, and renal biopsy should be performed if possible, avoiding the ignorance of renal damage caused by AA.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Vanherweghem JL, Depierreux M, Tielemans C, et al. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet. 1993;341(8842):387–391
- Reginster F, Jadoul M, van Ypersele de Strihou C. Chinese herbs nephropathy presentation, natural history and fate after transplantation. Nephrol Dial Transplant. 1997;12(1):81–86
- Hu WX, Liu ZH, Chen Z, et al. Clinical and pathological features in patients with renal injury associated with Aristolochia manshuriensis Kom. Chin J Nephrol Dial Transplant. 2003;12(6):504–511
- Priestap HA, Torres MC, Rieger RA, et al. Aristolochic acid I metabolism in the isolated perfused rat kidney. Chem Res Toxicol. 2012;25(1):130–139
- Qiu Q, Liu ZH, Chen HP, et al. Long-term outcome of acute renal injury induced by Aristolochia manshuriensis Kom in rats. Acta Pharmacol Sin. 2000;21(12):1129–1135
- Cui M, Liu ZH, Qiu Q, et al. Tumour induction in rats following exposure to short-term high dose aristolochic acid I. Mutagenesis. 2005;20(1):45–49
- Yang L, Li X, Wang H. Possible mechanisms explaining the tendency towards interstitial fibrosis in aristolochic acid-induced acute tubular necrosis. Nephrol Dial Transplant. 2007;22(2):445–456
- Yang L, Su T, Li XM, et al. Aristolochic acid nephropathy: variation in presentation and prognosis. Nephrol Dial Transplant. 2012;27(1):292–298
- Danhua W, Lihua S, Guofang D, et al. Kidney injury induced by mutong in newborns. Chin J Pediatr. 2000;38(6):392–393
- Chen W, Shen Y, Li A, et al. Clinical and pathological features of aristolochic acid nephropathy. Natl Med J China. 2001;81(18):1101–1105
- Lo SH, Mo KL, Wong KS, et al. Aristolochic acid nephropathy complicating a patient with focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2004;19(7):1913–1915
- Wang Y, Fu W, Wang H, et al. Renal microvascular injury in chronic aristolochic acid nephropathy and protective effects of Cozaar. Ren Fail. 2012;34(1):60–67
- Martinez MC, Nortier J, Vereerstraeten P, et al. Progression rate of Chinese herb nephropathy: impact of Aristolochia fangchi ingested dose. Nephrol Dial Transplant. 2002;17(3):408–412
- Vanherweghem JL, Abramowicz D, Tielemans C, et al. Effects of steroids on the progression of renal failure in chronic interstitial renal fibrosis: a pilot study in Chinese herbs nephropathy. Am J Kidney Dis. 1996;27(2):209–215
- Chen B, Bai Y, Sun M, et al. Glutathione S-transferases T1 null genotype is associated with susceptibility to aristolochic acid nephropathy. Int Urol Nephrol. 2012;44(1):301–307