Abstract
Background: End stage renal disease (ESRD) is a worldwide devastating health problem due to its increased prevalence in the population and high association with several pathologic conditions including immunodeficiency, which makes a significant contribution to morbidity and mortality. Aim: The present study aimed at analysis of T and B lymphocyte subpopulation and the detection of flowcytometric apoptosis markers on peripheral B and T lymphocytes in a cohort of children with ESRD. Subjects and methods: A case–control study was conducted on 28 children with ESRD. In addition, 30 age and sex matched healthy children were included as a control group. We used Annexin V-FITC binding assay as a sensitive probe for identifying cells undergoing apoptosis. Results: Circulating neutrophils, T and B lymphocytes were lower in patient group. In addition, apoptotic B and T lymphocytes occurred more frequently in children with ESRD than in the control group. Conclusion: Our finding of low numbers of circulating neutrophils, T and B lymphocytes, and increased portion of apoptotic B and T lymphocytes in children with ESRD, may emphasize the fact that these derangements are the main mechanisms responsible for the impairment of the immune system in ESRD children, also it adds to the fact that both cellular and humoral immunity affected in ESRD children. Finally, uremia and increased peripheral lymphocyte apoptosis were the major causes of lymphocyte populations' depletion in our ESRD patients.
Introduction
Chronic kidney disease (CKD) refers to a condition with irreversible kidney damage that can progress to end stage renal disease (ESRD). ESRD is defined according to the National Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI); as glomerular filtration rate being less than 15 mL/min per 1.73 m2.Citation1,Citation2 ESRD is associated with abnormalities of the immune system, which is evident by systemic inflammation, and immune deficiency. Systemic inflammation contributes to cardiovascular disease, cachexia, lipid abnormalities, malnutrition and anemia. The immune deficiencies expressed in the form of reduced granulocyte and monocyte/macrophage phagocytic function, defective antigen presentation by monocyte/macrophages, decreased antibody production by B lymphocytes and impaired T-cell mediated immunity. This immune deficiencies lead to increased susceptibility and severity of microbial infections, low response to vaccines, increased incidence of tumors and delayed hypersensitivity. The exact mechanisms responsible for these derangements are not fully understood. It may be due to causes related both to the disease (e.g., uremic toxins, increased oxidative stress, anemia, and malnutrition) and to the renal replacement therapy.Citation2–5 In most studies that had been conducted in dialysis patients, it is not clear whether these complications are due to the dialysis procedures or uremia per se. Few studies were conducted on pre-dialyzed ESRD patients had shown increases in pro-inflammatory cytokines and changes in leukocyte activation, indicating that dialysis alone is not responsible for alterations in the inflammatory state.Citation6,Citation7 A number of immunological theories and studies on lymphocyte subpopulation and lymphocyte functions have been carried out on adult patients with ESRD to identify any abnormal T and B cell clones and to investigate its possible role in immune deficiencies.Citation2,Citation4,Citation5 A small number of studies in children with ESRD suggested an altered inflammatory and immunological state.Citation3,Citation8 Since apoptosis plays an important role in lymphocyte homeostasis, the aim of this study was an analysis of lymphocyte subpopulation and detection of apoptosis in the peripheral B and T lymphocytes through flow cytometry in a cohort of children with ESRD on maintenance hemodialysis (HD).
Patients and methods
Patients
This was a case–controlled study carried out on 28 stable children with ESRD maintained on HD for an average of 38.5 months (12–113 months). The age range was 4–16 years, with a mean of 8.70 ± 3.69 years. ESRD individuals with evidence of acute or chronic infection, autoimmune disease, malignancy or previous radiation therapy, hematological disorders and those receiving antiviral, cytotoxic, corticosteroids or immunosuppressive medications were excluded from the study. All patients were negative for circulating hepatitis B surface antigen, hepatitis C, and HIV antibodies. Additionally, 30 healthy children were included as age- and sex-matched controls. The study was approved by the Institutional Review Board of the Faculty of Medicine, Assiut University, and informed consent was obtained from the guardians of both the patients and the controls.
Methods
All patients were subjected to the following: thorough medical history and examination including; age, sex, and duration of symptoms, blood pressure. HD therapy was performed thrice weekly using cellulose triacetate dialyzers.
Collection of blood samples and processing
In all ESRD patients, whole blood was collected from the vascular access prior to the initiation of dialysis. The blood samples were obtained by a syringe, applying gentle aspiration to minimize shear stress. Blood samples from the control individuals were collected from a peripheral vein in the same manner.
Routine monthly laboratory data were recorded. The complete blood count was done on Celltac E automated hematology analyzer (Nihon Khoden Corporation, Tokyo, Japan). Plasma biochemical measurements were obtained using standard laboratory methods.
Flow cytometric detection of T and B-lymphocyte subsets
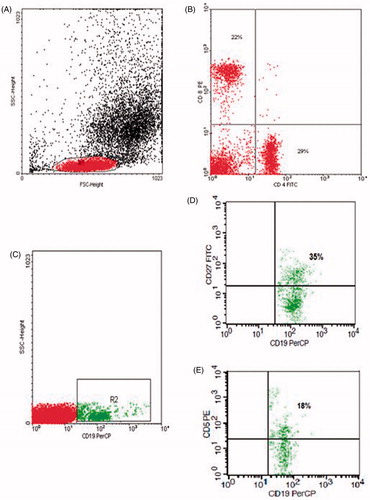
For detection of B-lymphocytes and T- lymphocytes, 50 µL of blood sample was stained with 5 µL of fluoro isothiocyanate (FITC)-conjugated CD4, phycoerythrin (PE)-conjugated CD8, pyridinium-chlorophyll-protein (Per-CP)-conjugated CD3 in one tube. Per-CP-conjugated CD19, PE-conjugated CD5 and FITC-conjugated CD27 in another tube, all Monoclonal antibodies were purchased from Becton Dickinson (BD) Biosciences (San Jose, CA). After incubation for 15 min at room temperature in the dark, red blood cells lysis was done. After one wash, the cells were resuspended in phosphate buffer saline (PBS). Flow cytometric analysis was done by FACSCalibur flow cytometry with Cell Quest software (BD Biosciences, San Jose, CA). Anti-human IgG was used as an isotype-matched negative control for each sample. Forward and side scatter histogram was used to define the lymphocyte population. Then, the percentages of CD19+ (B-lymphocytes), CD19+, CD27+ (memory B cells), CD19+, CD5+ (innate B1 cells), CD19+, CD5− (conventional B2 cells), CD3+ (T-lymphocytes), CD4+ (T-helper cells) and CD8+ (T-cytotoxic) were assessed in the lymphocyte population ().
Figure 1. Flow cytometric analysis of lymphocyte populations: (A) Forward and side scatter histogram was used to define the lymphocyte population (R1). (B) The expression of B and T cell markers were assessed in the lymphocyte population as CD4+ and CD8+ compared with the negative isotype control (not shown). (C) CD19+ cells were gated. (D, E) The expression of CD5 and CD27 in B cells was detected.
Flow cytometric detection of apoptosis of B and T lymphocytes
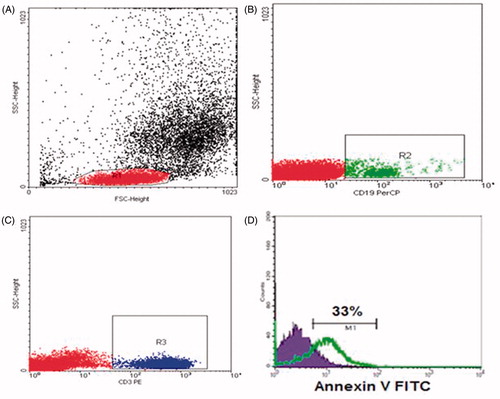
Apoptosis was measured by the Annexin V- fluoro isothiocyanate (FITC) binding assay according to the manufacturer's instructions (BD Biosciences). A total of 50 μL of whole blood were stained with 5 μL of Per-CP-conjugated anti-CD19 antibody and PE-conjugated CD3, washed twice with 2 mL of PBS and red blood cells were lysed. The cells were washed and resuspended in 100 μL of the Annexin V-conjugate binding buffer to which 5 μL of FITC-conjugated Annexin V was added. The mixture was incubated in a dark at room temperature for 15 min, after which 400 μL of the binding buffer was added and 10,000 cells were acquired and analyzed by FACSCalibur flow cytometry. Fl2 and FL3 channels were used to gate CD3+ T cells and CD19+ B cells, respectively. Then, the expression of Annexin V in T cells and B cells was detected ().
Figure 2. Flow cytometric detection of apoptosis of B and T lymphocytes: (A) Forward and side scatter histogram was used to define the lymphocyte population (R1). (B and C) CD19+ B cells and CD3+ T cells were gated. (D) Annexin V expression on lymphocyte populations. The positivity was defined as fluorescence (grey (green) histogram) higher than that of the isotype control (black (blue) histogram).
Statistical analysis
Data analysis was performed with the Statistical Package for Social Sciences (SPSS version 16, Chicago, IL). Due to the small sample size and a propensity for outliers in some of the variables, statistical differences between the groups were examined using the Mann–Whitney test. A value of p ≤ 0.05 denoted a statistically significant difference.
Results
Clinical data and biochemical characteristics of patients with ESRD and controls are summarized in . The mean age of the patients was 8.7 ± 3.69 years. The underlying causes of ESRD were obstructive uropathy in 10 patients (∼36%), reflux nephropathy in seven patients (25%%), nephritic syndrome in seven patients (25%), hypoplastic kidneys in one patient (3.50%), polycystic kidney disease in one patient (3.50%), and unknown etiology in two cases (∼7%).
Table 1. Baseline clinical and biochemical characteristics of patients with ESRD and controls.
As anticipated, serum creatinine and blood urea nitrogen concentrations were significantly higher in the ESRD children compared to the control group. In addition, serum concentration of phosphorus was significantly elevated in the ESRD group. Blood hemoglobin concentration was significantly lower, whereas platelet count, serum albumin, and calcium concentrations in the ESRD children were not significantly different from those observed in the control group (). As observed in , the numbers of neutrophils, lymphocytes and monocytes were significantly lower in the ESRD patients as compared to the control group. However, the total white blood cell counts were not significantly different from the control group.
Table 2. Leukocyte count and lymphocyte subsets in patients with ESRD and controls.
T-lymphocyte and B-lymphocyte subset data
As shown in , the mean number of circulating CD3 T-lymphocytes was significantly decreased in patients compared to healthy control (p < 0.05). Also, the number of CD4 cells and the ratio of CD4 to CD8 cells were significantly reduced in the ESRD group (p = 0.001). However, CD8 T cells in the ESRD group were not significantly different from those observed in the control group.
Among B cell subsets; there was a significant decrease in the number of total B cells (CD19+), innate B1 cells (CD19+ CD5+), conventional B2 cells (CD19+ CD5−), and Memory B cells (CD19+ CD27+) observed in ESRD children, as compared to healthy controls ().
Apoptosis data
Compared to the controls, the ESRD patients exhibited a significant increase in the percentage of both apoptotic B and T lymphocytes ().
Table 3. Apoptosis of B lymphocytes and T lymphocytes in patients with ESRD and controls.
Discussion
Our ESRD children showed a significantly lower number of neutrophils and monocytes as compared to healthy controls. In previous studies in children and adults with uremia, abnormalities in absolute neutrophil and monocyte counts were reported.Citation9–11 Nairn et al.Citation9 reported that children with severe chronic renal failure (CRF) showed significantly reduced total white cell count, absolute neutrophil and monocyte counts.
Among T-cell populations; there were significantly decreased number of circulating CD3, CD4 cells and reduced ratio of CD4 to CD8 cells in our ESRD children. In addition, a significantly increased portion of T cells undergoing apoptosis. The T-cell populations' depletion in our study could be explained by the effect of uremia and increased peripheral lymphocyte apoptosis.
Our results are consistent with that of previous studies,Citation2,Citation3,Citation10,Citation12,Citation13 that reported that ESRD patients have a significant decrease in T-lymphocytes populations.
Bouts et al.Citation10 reported that total lymphocytes and lymphocyte subsets were reduced in children on dialyzed and non-dialyzed renal failure groups compared with controls. Matsumoto et al.Citation12 observed an increase in the apoptosis of T lymphocytes from both dialyzed and undialyzed uremic adult patients. Furthermore, in vivo, uremic T lymphocytes expressed Fas with a higher intensity than control T cells, suggesting that uremic T cells undergo apoptosis. Borges et al.Citation2 found that ESRD patients showed a decrease in the total circulating CD3+ lymphocytes, especially in CD4+ T cells, and a higher proportion of T lymphocytes in the latest stage of apoptosis and a higher proportion of apoptotic CD4+ T cells observed in the patients immediately after HD procedure. Meier et al.Citation13 reported that T cell apoptosis increased by 2.4-fold in chronic HD patients compared with 1.8-fold in patients with ESRD and only 1.2-fold in controls. This may account for the T lymphopenia, progressive immunodeficiency, and increased infection risk seen in ESRD patients.Citation13
Among B-cell populations in our study; there were significantly decreased number of total B cells, innate B1 cells, conventional B2 cells and memory B cells in children with ESRD. In addition, a significantly increased portion of B cells undergoing apoptosis. B cell lymphopenia in ESRD patients may be due to increased susceptibility to apoptosis.
The diversity of the B-lymphocytes determines the individual’s capacity to mount a protective immune response. Innate B1 cells account for 25–27% of peripheral blood B lymphocytes and produce mainly IgM antibodies that have low-affinity and high cross-reactivity properties.Citation14,Citation15 These antibodies fight a variety of infections prior to the production of high-affinity-specific antibodies. Conventional B2 cells (CD5− B cells), account for 75–80% of peripheral blood B lymphocytes and produce a more diverse array of antibodies with high-affinity interactions.Citation15,Citation16 When the mature B lymphocytes recognize antigen, they differentiate into long-lived memory B cells (CD27+) and plasma cells that produce and secrete antigen-specific antibodies. Memory cells can survive decades. Upon a subsequent exposure to antigen, memory B cells respond rapidly by producing antibodies with a high affinity for the given antigen. In adult humans, about 40% of all circulating B cells are memory B cells.Citation17,Citation18
Our results are matched previous studies,Citation4,Citation10,Citation19,Citation20 that documented a reduced number of B lymphocytes of ESRD patients. In addition, they have a higher rate of apoptosis than healthy controls. Bouts et al.Citation10 reported lower B-cell count in children on dialyzed and non-dialyzed renal failure patients compared with controls. Pahl et al.Citation4 found that populations of innate B1, memory B-cell, and conventional B2 cell subsets were markedly reduced in the ESRD patients as compared to the corresponding values in the normal control group. In contrast to the present study, they found no difference in the proportions of apoptotic B cells among the ESRD patients and control subjects. This difference could be explained by the high sensitivity of Annexin V that we used in our study.
Bouts et al.Citation19 found reduced memory B cell numbers in dialyzed children with ESRD and children with CRF before starting dialysis treatment compared to healthy controls. Fernandez-Fresnedo et al.Citation20 found peripheral blood B-cell lymphopenia and higher rate of apoptosis of B lymphocytes in uremic patients than in the healthy controls.
Conclusion
ESRD patients exhibited a marked reduction of T and B lymphocytes. Increased lymphocyte apoptosis may be the major cause of their depletion in ESRD patients.
Declaration of interest
The authors declare no conflicts of interests. The authors alone are responsible for the content and writing of this article.
References
- Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147
- Borges A, Borges M, Fernandes J, et al. Apoptosis of peripheral CD4 (+) T-lymphocytes in end-stage renal disease patients under hemodialysis and rhEPO therapies. Ren Fail. 2011;33(2):138–143
- Szczepánska M, Szprynger K, Mazur B, Szczepánski T. Alpha beta and gamma delta T cell subsets in chronic renal failure in children on dialysis treatment. Pediatr Int. 2002;44(1):32–36
- Pahl MV, Gollapudi S, Sepassi L, Gollapudi P, Elahimehr R, Vaziri ND. Effect of end-stage renal disease on B-lymphocyte subpopulation, IL-7, BAFF and BAFF receptor expression. Nephrol Dial Transplant. 2010;25(1):205–212
- Vaziri ND, Pahl MV, Crum A, Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr. 2012;22(1):149–156
- Girndt M, Sester U, Sester M, Kaul H, Kohler H. Impaired cellular immune function in patients with end-stage renal failure. Nephrol Dial Transplant. 1999;14:2807–2810
- Pereira BJ, Shapiro L, King AJ, Falagas ME, Strom JA, Dinarello CA. Plasma levels of IL-1 beta, TNF alpha and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney Int. 1994;45:890–896
- Zwolinska D, Medynska A, Szprynger K, Szczepanska M. Serum concentration of IL-2, IL-6, TNF-alpha and their soluble receptors in children on maintenance hemodialysis. Nephron. 2000;86:441–446
- Nairn J, Hodge G, Henning P. Changes in leukocyte subsets: clinical implications for children with chronic renal failure. Pediatr Nephrol. 2005;20:190–196
- Bouts AH, Out TA, Schroder CH, et al. Characteristics of peripheral and peritoneal white blood cells in children with chronic renal failure dialyzed or not. Perit Dial Int. 2000;20:748–756
- Moser B, Roth G, Brunner M, et al. Aberrant T cell activation and heightened apoptotic turnover in end-stage renal failure patients: a comparative evaluation between non-dialysis, hemodialysis, and peritoneal dialysis. Biochem Biophys Res Commun. 2003;308:581–585
- Matsumoto Y, Shinzato T, Amano I, et al. Relationship between susceptibility to apoptosis and Fas expression in peripheral blood T cells from uremic patients: a possible mechanism for lymphopenia in chronic renal failure. Biochem Biophys Res Commun. 1995;215:98–105
- Meier P, Dayer E, Blanc E, Wauters JP. Early T cell activation correlates with expression of apoptosis markers in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:204–212
- Kipps T. The CD5 B cell. Adv Immunol. 1989;47:117–185
- Herzenberg L, Haughton G, Rajewsky K. CD5 B cells in development and disease. Ann NY Acad Sci. 1992;651:591–601
- Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol. 2001;13:195–201
- Uckun F. Regulation of human B-cell ontogeny. Blood. 1990;76:1908–1923
- Gray D. Immunological memory: a function of antigen persistence. Trends Microbiol. 1993;1:39–41
- Bouts AH, Davin JC, Krediet RT, et al. Children with chronic renal failure have reduced numbers of memory B cells. Clin Exp Immunol. 2004;137(3):589–594
- Fernandez-Fresnedo G, Ramos MA, Gonzalez-Pardo MC, de Francisco AL, Lopez-Hoyos M, Arias M. B lymphopenia in uremia is related to an accelerated in vitro apoptosis and dysregulation of Bcl-2. Nephrol Dial Transplant. 2000;15:502–510

