Abstract
Objectives: The purpose of this study was to determine if intra-abdominal pressure (IAP) could predict acute renal injury (AKI) in the postoperative period of abdominal surgeries, and which would be its cutoff value. Patients and methods: A prospective observational study was conducted in the period from January 2010 to March 2011 in the Intensive Care Units (ICUs) of the University Hospital of Botucatu Medical School, UNESP. Consecutive patients undergoing abdominal surgery were included in the study. Initial evaluation, at admission in ICU, was performed in order to obtain demographic, clinical surgical and therapeutic data. Evaluation of IAP was obtained by the intravesical method, four times per day, and renal function was evaluated during the patient’s stay in the ICU until discharge, death or occurrence of AKI. Results: A total of 60 patients were evaluated, 16 patients developed intra-abdominal hypertension (IAH), 45 developed an abnormal IAP (>7 mmHg) and 26 developed AKI. The first IAP at the time of admission to the ICU was able to predict the occurrence of AKI (area under the receiver-operating characteristic curve was 0.669; p = 0.029) with the best cutoff point (by Youden index method) ≥7.68 mmHg, sensitivity of 87%, specificity of 46% at this point. The serial assessment of this parameter did not added prognostic value to initial evaluation. Conclusion: IAH was frequent in patients undergoing abdominal surgeries during ICU stay, and it predicted the occurrence of AKI. Serial assessments of IAP did not provided better discriminatory power than initial evaluation.
Introduction
The increase in intra-abdominal pressure (IAP) is a clinical condition developed by approximately half of Intensive Care Unit (ICU) patients.Citation1 In patients undergoing abdominal surgeries an elevated IAP may be explained by intraoperative administration of large amounts of fluids causing increased capillary permeability and visceral edemaCitation2–4 and its prevalence ranges from 33 to 41%.Citation5,Citation6
Values between 5 and 7 mmHg for IAP are considered normal in critical patientsCitation2,Citation3,Citation7 while values above 7 mmHg are considered abnormal.Citation2 The presence of two consecutive values above 12 mmHg characterizes the Intra-Abdominal Hypertension (IAH) that in extreme values constitutes the Abdominal Compartment Syndrome (ACS), defined by an IAP >20 mmHg associated to the organic dysfunction.Citation3,Citation8–13 Increased IAP is an important cause of morbidity and mortality in critically ill patients with consequent pulmonary, hepatic, central nervous and renal system impairments.Citation3,Citation7,Citation10,Citation14
Acute kidney injury (AKI) is defined by a reduction in renal function, expressed by an increased of serum creatinine levels or oliguria for over 6 h, regardless adequate fluid replacement.Citation14,Citation15 This clinical condition affects 5–45% of ICU patients and implies in increased mortality rate, nearly 50%.Citation16,Citation17
Clinical and experimental studies reported that high levels of IAP are predictors of AKI. The association between IAP and oliguria has started being reported at the end of the nineteenth century, however, only recently have the effects of increased IAP on renal functions been recognized.Citation10
The increase in IAP is rarely diagnosed in ICU Citation10 and the lack of diagnosis of this condition may lead to the worsening of patient prognoses because of retardation of appropriate interventions.Citation18
The current literature shows conflicting cutoff values of IAP to predict AKI, possibly due to the fact that many studiesCitation5,Citation6,Citation10 were conducted before publishing of the first Consensus of IAH/ACS, which standardized the measurement method of IAP.Citation2 So, further studies based on that consensus are required in critical patients after abdominal surgeries. Once there is no Brazilian data with this purpose, this study aimed to verify the discriminatory power of IAP on prediction of AKI, and its best cutoff value between patients in the postoperative (PO) period of abdominal surgery.
Patients and methods
A prospective observational study was conducted in the period from January 2010 to March 2011 in the Intensive Care Unit of the University Hospital of Botucatu Medical School. This study was approved by the Research Ethics Committee (protocol: 3364/2009). All participants or legal representatives signed an Informed Consent Form.
The inclusion criteria were patients aged over 18 years, undergoing abdominal surgery. Patients were excluded when they were diagnosed with chronic renal failure on dialysis or contraindications for measurement of IAP as bladder injury or pelvic trauma. Patients who had notoriouslyCitation2 a chronic increase in IAP were also excluded, namely pregnant women and obese with body mass index (BMI) >32 kg/m2. Patients with early death were also excluded. Death in a period of less than 24 h after ICU admission was considered early. There were included only open surgeries and lasting longer than 120 min, because these patients needed indwelling urinary catheter (IUC).
The following data were collected at the patient’s admission: age, sex, race, diagnoses, pre-existing co-morbidities, date of hospital and ICU admission. Clinical characteristics recorded included: Acute Physiological and Chronic Health Evaluation II (APACHE II), BMI, presence of Systemic Inflammatory Response Syndrome (SIRS), septic shock or presence of intra-abdominal or retroperitoneal tumors.
SIRS was considered when two or more of the following variables were present over 24 h: body temperature >38 °C or <36 °C, heart rate (HR) >90/min, respiratory rate (RR) >20 rpm or PaCO2 <32 mmHg, Leukogram <4 × 109 cells/L or >12 × 109 cells/L, or >10% of immature forms. Septic shock was considered when SIRS was present and associated with an infectious focus, and Mean arterial pressure (MAP) <90 mmHg refractory to the volume.Citation19
With respect to the data of surgery were recorded the type of procedure, duration, type of catheter and anesthesia. Administrated drugs also were daily recorded as diuretics, sedative-hypnotic drugs, vasoactive agents, corticoids and antibiotics. Laxatives or rectal enema preoperatively were not employed to any patient evaluated except for colon surgery in which all patients have been cleaned their colons. No patient was included in the study making use Hemovac drain.
Daily measurement of IAP was performed using the original method of Kron et al.Citation20 updated by Malbrain et al.Citation2 The IAP was measured every 6 h, counting from the time of admission in the ICU until discharge from the unit, death or the occurrence of AKI. Patients were admitted at the ICU after surgery where were kept with catheter indwelling urinary three-way. This procedure was connected to the equipment of electronic transducer-based pressure. Then patients were kept in complete supine position to receive an infusion volume of 25 mL of 0.9% sodium chloride and the measurement system was zeroed at the axillary midline. The value of IAP was measured at end-expiration and expressed in mmHg. After evaluation, the infused volume was subtracted from the urinary volume output recorded at that time.
IAP was measured at the patient’s admission at the ICU (IAP1). At the same time, evaluations of MAP (non-invasive), heart rate and respiratory rate were performed. Mean IAP was considered as mean of values recorded in the first day, and maximum IAP as the highest daily value.
Renal function was also evaluated in the same period according to the Acute Clinical Practice Guidelines for AKI by a detailed monitoring of hourly urinary output and daily laboratory analyses of creatinine. AKI was diagnosed in presence of an increase in serum creatinine levels ≥0.3 mg/dL within 48 h or increase ≥1.5 times basal level within the previous seven days, or decreased of urinary volume ≤0.5 mL/kg/h over 6 h.Citation21 IAH was defined when the patient had two consecutive measures of IAP ≥12 mmHg.Citation2 Patients were followed until ICU discharge, death or the occurrence of AKI.
Continuous and parametric variables were expressed as mean ± standard deviation, continuous and non-parametric variables were expressed as median and interquartile range, and the categorical variables were expressed in absolute number and percentage. The receiver-operating characteristic (ROC) curve was drawn in order to detect the optimal cutoff point as well as the area under the curve and 95% confidence interval (CI 95%). The ROC curve provides information on the tradeoff between sensitivity and specificity for each cutoff point of and index. The optimal cutoff point for IAP was considered as the highest combined value of sensitivity and specificity: “Youden index”.Citation22,Citation23 The groups were compared by chi-square for categorical variables, by “t” test for normally distributed variables and Mann–Whitney test for non-normally distributed variables. Variables that differ statistically between groups were selected to compose a multiple logistic analysis model. The p-value of 0.05 was considered statistically significant.
Results
There were evaluated 78 consecutive patients. Eighteen were excluded (23.3%): because of early death (seven patients), ICU stay less than 24 h (10 patients) and traumatic brain injury (one patient). After exclusion, the 60 remaining patients were evaluated for 1 d, 34 patients remained in the ICU for 2 d, and 20 patients for 3 d. The patients were predominantly male (57%) and white (76.6%), aged 59.7 ± 15.6 years.
Surgeries of gut or gall bladder and liver corresponded to 48.3% and 20% of the sample, respectively. The median total surgical time was 210 min (127–312) and 36 patients (60%) underwent emergency or urgent surgery. APACHE II score of severity index of the patients, calculated at admission to the protocol, had a mean value of 12.91 ± 5.28 and BMI mean was 25.50 ± 4.96 kg/m2. Diagnosis of intra-abdominal or retroperitoneal tumor was found in 29 patients (48.3%). Considering crystalloids, colloids, mannitol, medications, blood components and diet, the mean volume received at admission to the protocol was 7.6 ± 3:37 L in the first day, and urinary output was 2.7 ± 1.94 L in the first day. Among the patients, 25 (41.6%) were admitted to the study of invasive ventilatory support. The highest and the lowest daily mean value of MAP at admission was 90 ± 13.6 mmHg and 82 ± 16.0 mmHg, respectively. SIRS was found in 28 patients (46.6%) and septic shock in 23 patients (38%), the remaining ones did not show neither the referred clinical situations, nor met the criteria for sepsis or severe sepsis. Medications used by patients, in the first day of the study, were vasoactive drugs for 25 patients (41.66%); sedative or analgesic or hypnotic medication for 22 patients (37%); diuretic medication for 10 patients (17%) and corticoids for eight patients (13%). A total of 35 (58%) evaluated patients received blood transfusion.
Among the evaluated patients, 26 developed AKI (incidence of 43%). Mean IAP of the patients who developed and did not develop AKI was 11.0 ± 4.51 mmHg and 8.8 ± 4.98 mmHg, respectively (p = 0.09).
The first IAP in the first day of the study was able to predict the development of AKI in this population (p = 0.029) with significant discriminating power by the ROC curve (), with an area under the curve of 0.669, CI 95% = 0.529–0.803. Sensitivity and specificity were 87% and 46%, respectively, and the optimal cutoff point was ≥7.68 mmHg. Considering mean and maximum values, serial assessment of IAP in the first PO did not show superiority over the evaluation alone for prediction of AKI ().
Figure 1. ROC curve presenting the first intra-abdominal pressure in the first day in regard of acute kidney injury development; AUC: area under ROC curve; CI: confidence interval.
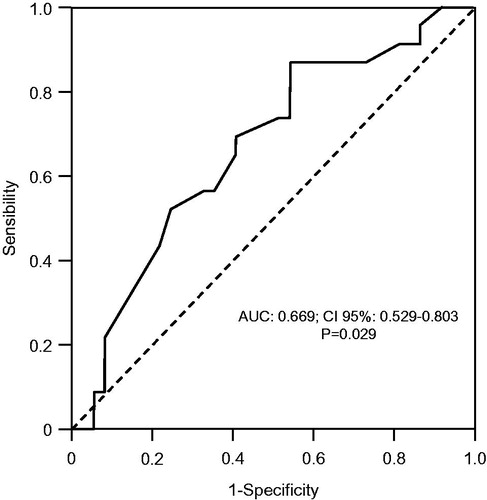
Table 1. Serial evaluation of IAP, considering average and maximum values for the prediction of AKI.
Clinical data comparing the two groups (AKI and non-AKI groups) are showed in . The groups differed only in regard of age 65 ± 13.7, and 56 ± 16.1 years (p = 0.025), APACHE II 14 ± 6 and 11 ± 4 (p = 0.024), IAH frequency 11 (42%) and five (19%) (p = 0.035) and urinary output 1.7 ± 0.87 and 3.3 ± 2.2 (p = 0.001). There were 16 patients under mechanical ventilation and 15 patients without ventilation support at the IAP assessment, however occurrence of AKI was not different between them (p = 0.0281). A logistic regression was performed with these variables, except urinary output (that is a criterion to AKI). IAH occurrence was associated with AKI even when adjusted for APACHE II and age ().
Table 2. Clinical characteristics in according to occurrence of acute kidney injury.
Table 3. Association between IAH and AKI in patients submitted to abdominal surgery.
Discussion
In the last 20 years, the interest in IAH and ACS has risen. That importance is attributed to the awareness of the occurrence of organic dysfunctions, and as a consequence, an increased mortality caused by an increase in IAP.Citation3,Citation10,Citation13 AKI is a common occurrence in ICU patients and it predicts an ominous prognosis. Some studies have reported that increased IAP is associated with increased frequency of AKI.Citation2,Citation3,Citation6,Citation9,Citation10,Citation12,Citation13,Citation19,Citation24,Citation25 However, in the best of our knowledge, no Brazilian study reported the predictive value of routine measurement of IAP to further development of AKI. Therefore, the objective of this study was to evaluate prospectively the association between increased IAP and AKI in the PO period of abdominal surgery in a Brazilian ICU. In the present study, the incidence of IAH was 27% and a statistically significant association was found between level of IAP and AKI, which corroborates the findings in the literatureCitation2,Citation3,Citation4,Citation5,Citation6,Citation9,Citation10,Citation11–13,Citation19,Citation26 As the first measurement of IAP at ICU admission revealed a discriminating power similar to that of the 24 hour follow-up, this finding contributes to highlight the importance of early detection of changes in abdominal pressureCitation20,Citation35 to enable clinical interventions.Citation3,Citation13
An epidemiological multicenter study evaluated 14 ICUs in six different countries including Brazil and the occurrence of IAH was 27%.Citation11 However, that study evaluated not only PO of abdominal surgeries and do not evaluated specifically the renal consequences of IAH. Concerning the incidence of IAH in the PO period of abdominal surgeries, it ranges from 4 to 41%.Citation27,Citation28–34 Moreover, another study reported an incidence of 23% of primary IAH (associated with injury or disease in the pelvic abdominal region) in intensive care patients.Citation26
Some epidemiological studies had been conducted before the first Consensus of IAH/ACS, and this fact could explain the variation in the incidence reported by them, due to lack of standardization of measurements, as they used infusion volumes higher than those recommended nowadays (overestimating IAP value), and the incorrect definition of IAH.Citation5,Citation6,Citation24,Citation35,Citation34 Despite being standardized at Consensus that IAP over 12 mmHg in two daily and consecutive measurements is optimal to define IAH, the use of pressure values in which an organic dysfunction is evident is also appropriate for the same definition.Citation2,Citation3,Citation10,Citation13
One of the major complications arising from IAH/ACS is AKI. In the current study, the diagnosis of AKI was recorded in 43.3% of the sample population using the criterion by AKIN (Acute Injury Network), which is in accordance with previous studies related to AKI incidence.Citation14–17,Citation36 Furthermore, in the present study, IAP was able to predict the development of AKI in the study population. In a study that demonstrated the relationship between IAH and AKI, the occurrence of primary IAH at admission to the protocol was 27%,Citation10 which is similar to our findings.
Kron et al. reported that the increase in IAP is related to the decrease in the urinary output, and that the surgical decompression is able to reverse this situation,Citation19 while Sugrue et al. reported that IAH (defined as IAP >20 mmHg) was associated with AKI in ICU patients following abdominal surgery.Citation6 Later, the same group conducted another study with similar objectives, but considering IAH values over 18 mmHg as independent predictor of AKI. Moreover, they used PO serum creatinine levels ≥1.47 mg/dLCitation5 as the criterion for AKI diagnosis. Differently from that study, we used AKIN as the diagnostic criterion, enabling earlier detection of AKI.Citation37
Differently from findings reported by Dalfino et al.,Citation10 the analysis performed by our group, with the first IAP measured at the admission to the ICU after the surgical procedure, showed significant discriminating power to predict AKI, with the optimal cutoff point of 7.68 mmHg. However, their study associated IAH and AKI, and the optimal cutoff point was found to be >12 mmHg. It is important to point out that Dalfino et al. considered the highest IAP, and the occurrence of AKI was defined by RIFLE criterion.Citation10 It should also be pointed out that IAP for prediction of AKI had better sensibility than specificity in the present study and in the study of Dalfino et al.Citation10
In the present study, the similarity among results obtained by the ROC curve comparing IAP values at different moments suggests that measurement of IAP at the patient’s admission at the ICU after the surgical procedure is efficient to predict AKI. This finding strengthens the need for early diagnosis of increased IAP, so that clinical or surgical interventions might be performed to control or reduce it, therefore preventing its deleterious effects.Citation2,Citation3,Citation13
It should be considered that the prognostic power of abdominal hypertension, with a cutoff value of IAP>12 mmHg had a sensitivity of 43% and specificity of 84% in the prediction of AKI. Thus, not intervene in situations IAP high, reaching 12 mmHg, may increase the risk of injury to several patients, therefore, the value 7.68 may be more appropriate for the prevention of AKI.
We could point out as the strengths of this study the prospective design and the fact that it is the first Brazilian local study to evaluate the discriminating power of IAP on the outcome of AKI. Furthermore, the method used for measurement of IAP was standardized and the guidelines from the last IAH/ACS consensus were followed. Routine evaluation of IAP has great importance in clinical practice, therefore, needs a reliable and feasible method of evaluation. The intravesical method, employed in this study, is a noninvasive and safe measurement used worldwide because of its ease and minimal cost.
Some limitations of this study should be recognized. The reduced number of patients included in the study could compromise the statistical power; however, the number of patients evaluated was sufficient to demonstrate the discriminatory capacity of IAP to predict AKI. Presence of auto PEEP (positive-end expiratory pressure) was not assessed but all the patients under mechanical ventilation had clinical conditions for weaning of ventilator at the IAP evaluation. Moreover the prevalence of AKI was not different between patients with or without invasive ventilation support. Besides, possible interventions of the routine of the ICU to reduce IAP, such as the use of prokinetic agents and performance of paracentesis, rectal enema and decompressive laparotomy have not been evaluated.
Conclusion
The results from this Brazilian case series showed that IAH was frequent in our ICU patients submitted to abdominal surgery in which the elevated IAP presented a good discriminatory power for the occurrence of AKI, however, serial assessments of IAP did not provide better discriminatory power than initial evaluation.
Declaration of interest
The authors declare no conflicts of interests. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors thank Gastric Surgery and Care Unit Service teams of the University Hospital, Botucatu Medical School and Dr. Hugo Hyung Bok Yoo for the confidence in their work.
References
- Malbrain MLNG, Chiumello D, Pelosi P, et al. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med. 2004;30:822–829
- Malbrain MLNG, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on intra-abdominal Hypertension and Compartment Syndrome. I Definitions. Intensive Care Med. 2006;32:1722–1732
- Cheatham ML, Malbrain MLNG, Kirkpatrick A, et al. Results from the international conference of experts on intra-abdominal hypertension and compartment syndrome. II Recommendations. Intensive Care Med. 2007;33:951–962
- Serpytis M, Ivaskevicius J. The influence of fluid balance on intra-abdominal pressure after major abdominal surgery. Medicina (Kaunas). 2008;44:421–427
- Sugrue M, Jones F, Bishop G, Bauman A, Hillman K. Intra-abdominal hypertension is an independent cause of postoperative renal impairment. Arch Surg. 1999;134:1082–1085
- Sugrue M, Buist MD, Hourihan F, Deane S, Bauman A, Hillman K. Prospective study of intra-abdominal hypertension and renal function after laparotomy. Br J Surg. 1995;82:253–258
- Torquato JÁ, Lucato JJJ, Antunes T, Barbas CV. Interaction between intra-abdominal pressure and positive-end expiratory pressure. Clinics. 2009;64:105–112
- Keulenaer BD, Waele JJ, Powell B, Malbrain MLNG. What is normal intra-abdominal pressure and how is it affected by positioning, body mass and positive end-expiratory pressure? Intensive Care Med. 2009;35:969–976
- De Waele J, Laett I, Kirkpatrick A, Hoste E. Intra-abdominal hypertension and abdominal compartment syndrome. Am J Kidney Dis. 2011;57:159–169
- Dalfino L, Tulio L, Donadio I, Malcangi V, Brienza N. Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med. 2008;34:707–713
- Malbrain MLNG, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri M. Incidence and prognosis of intra-abdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med. 2005;33:315–322
- Vidal MG, Ruiz Weisser J, et al. Incidence and clinical effects of intra-abdominal hypertension in critically ill patients. Crit Care Med. 2008;36:1823–1831
- De Waele J, Cheatham M, Malbrain M, Kirkpatrick A, Sugrue M, Balogh Z. Recommendations for research from the international conference of experts on intra-abdominal hypertension and abdominal Compartment syndrome. Acta Clin Belg. 2009;64:203–209
- Khalil P, Murthy P, Palevsky P. The patient with acute kidney injury. Prim Care Clin Office Pract. 2008;35:239–264
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients. JAMA. 2005;294:813–818
- Dennen P, Douglas IS, Anderson R. Acute kidney injury in the intensive care unit: an update and primer for the intensivist. Crit Care Med. 2010;38:268–275
- Balbi AL, Gabriel DP, Barsante RC, Caramori JT, Martin LC, Barretti P. Mortalidade e prognóstico específico em pacientes com insuficiência renal aguda. Rev Assoc Med Bras. 2005;51:318–322
- Malbrain M. Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Int Care Med. 2004;30:357–371
- Dellinger RP. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60
- Kron I, Harman K, Nolan S. The measure of intra-abdominal pressure as a criterion for re-exploration. Ann Sug. 1984;99:28–30
- Clinical practice guidelines for acute kidney injury; 2012. Available at: 2: http://www.kdigo.org/clinical_practice_guidelines/AKI.php
- Sackett D, Straus SE, Richardson WS, Rosemberg W, Haynes RB. Medicina baseada em evidências. 2003. 2aed. P89. Ed. ArtMed
- Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–657
- Bioncofiori G, Bindi ML, Romanelli AM, et al. Postoperative intra-abdominal pressure and renal function after liver transplantation. Arch Surg. 2003;138:703–706
- De Waele JJ, Lepaniemi AR. Intra-abdominal hypertension in acute pancreatitis. World J Surg. 2009;33:1128–1133
- Reintam A, Pam P, Kitus R, Kern H, Starkopf J. Primary and secondary intra-abdominal hypertension-different impact on ICU outcome. Intensive Care Med. 2008;34:1624–1631
- Sugrue M, Buhkari Y. Intra-abdominal pressure and abdominal compartment syndrome in acute general surgery. World J Surg. 2009;33:1123–1127
- Mayberry JC, Welker Jk, Goldman RK, Mullins RJ. Mechanism of acute ascites formation after trauma resuscitation. Arch Surg. 2003;138:773–776
- Platell CFE, Hall J, Clarke G, Lawrence-Brown M. Intra-abdominal pressure and renal function after surgery of the abdominal aorta. Aust NZ J Surg. 1990;60:213–216
- Aker DL, Fowl RJ, Kempezinski RF. Temporary closure of abdominal wall used rubber silicone after ruptured of aortic aneurysm. Vasc Surg J. 1991;14:48–51
- Meldrum DR, Moore FA, Moore EE, Franciose RJ, Sauaia A, Burch JM. Prospective characterization and selective management of theabdominal compartment syndrome. Am J Surg. 1997;174:667–673
- Ertel W, Oberholzer A, Platz A, Stocker R, Trentz O. Incidence and clinical pattern of the abdominal compartment syndrome after ‘‘damage-control’’ laparotomy in 311 patients with severe abdominal and/or pelvic trauma. Crit Care Med. 2000;28:1747–1753
- Schachtrupp A, Jansen M, Bertram P, Kuhlen R, Schumpelick V. Abdominal compartment syndrome: significance, diagnosis and treatment. Anaesthesist. 2006;55:660–667
- Cheatham M, White M, Sagraves S, Johnson J, Block E. Abdominal perfusion pressure: a superior parameter in the assessment of intra-abdominal hypertension. J Trauma. 2000;49:621–627
- Malbrain ML, De Laet IE, De Waele JJ. IAH/ACS: rationale for suveillance. World J Surg. 2009;33:1110–1115
- Uchino S, Bellomo R, Morimatsu H, et al. External validation of severity scoring systems for acute renal failure using a multinational database. Crit Care Med. 2005;33:1961–1967
- Brochard L, Abroug F, Brenner M, et al. Ad hoc committee on acute renal failure (2010) An Official ATS/ERS/ESICM/SCCM/SRLF Statement Prevention and Management of Acute Renal Failure in the ICU Patient: an international consensus conference in intensive care medicine. Am J Respir Crit Care Med. 2010;181:1128–1155

