Abstract
Association between endothelial nitric oxide synthase (eNOS) gene polymorphism and Henoch-Schönlein purpura (HSP)/Henoch-Schönlein purpura nephritis (HSPN) risk is still controversial. A meta-analysis was performed to evaluate the association between eNOS gene polymorphism and HSP/HSPN susceptibility. A predefined literature search and selection of eligible relevant studies were performed to collect data from electronic database. Three articles were identified for the analysis of association between eNOS gene polymorphism and HSPN/HSP risk. eNOS G894T gene polymorphism was not associated with HSPN susceptibility and the risk of patients with HSP developing into HSPN. Interestingly, eNOS G894T T allele and GG genotype were associated with HSP susceptibility, but not the TT genotype. eNOS T786C TT genotype was associated with HSPN susceptibility, but not C allele and CC genotype. Furthermore, eNOS T786C gene polymorphism was not associated with HSP risk and the risk of patients with HSP developing into HSPN. In conclusion, eNOS T786C TT genotype was associated with and eNOS G894T T allele and GG genotype were associated with HSP susceptibility. However, more studies should be performed in the future.
Introduction
Henoch-Schönlein purpura (HSP), characterized by immunoglobulin (Ig) A-predominant deposition in small blood vessels, is one of the most common vasculitis syndromes, and skin purpura, arthritis and gastrointestinal disease are the primary clinical performance.Citation1,Citation2 The condition is also accompanied by renal involvement, which is named Henoch-Schönlein purpura nephritis (HSPN).Citation1,Citation2 Some current investigationsCitation3–6 suggested that genetic factors might play a key role in the onset of HSP/HSPN.
Endothelial nitric oxide synthase (eNOS) is an enzyme constitutively expressed especially in endothelial cells, and is largely responsible for nitric oxide (NO) bioavailability at the endothelial level.Citation7 NO, a ubiquitous vasodilator, is an important regulator of renal sodium excretion, and reduced NO generation induces renal injury, and impairment of endothelial NO generation is considered the major deterioration factor for progressive renal disease.Citation8,Citation9
The present epidemiologic study shows that the eNOS gene polymorphism has been implicated in the etiology of HSP/HSPN. However, the available evidence reported to date is weak, due to sparseness of data or disagreements among studies. There was rare meta-analysis to explore the association of eNOS gene polymorphism with HSP/HSPN risk. We performed this meta-analysis from all English published reports to investigate the relation between eNOS gene polymorphism and HSP/HSPN susceptibility, with the intention to provide a much more reliable finding on the significance of the association.
Materials and methods
Search strategy
The relevant studies were screened from the search engines of PubMed, Embase, Cochrane Library, and CBM-disc (China Biological Medicine Database) as of 1 June 2014. The following terms in English were used to complete the search: “Henoch-Schönlein purpura”, “HSP”, “endothelial nitric oxide synthase”, “eNOS”, “polymorphism” and “variant”. We also extended search spectrum to the “related articles” and the bibliographies of all retrieved studies. If multiple publications of the same data from the same study group occurred, we only recruited the later paper for analysis.
Inclusion and exclusion criteria
Inclusion criteria: (1) A case-control study; (2) The outcome had to be HSP or HSPN; (3) There had to be at least two comparison groups (HSP or HSPN group vs. control group).
Exclusion criteria: (1) Review articles, editorials, and case reports. (2) Articles did not provide the detail genotype data; (3) Investigating the association of other genes with HSP or HSPN. (4) Investigating the role of eNOS to diseases. (5) Multiple publications of the same data from the same study group.
Data extraction and synthesis
The following information was extracted from each study independently by two investigators: first author’s surname, year of publication, ethnicity of study population, and the number of cases and controls for eNOS genotype. Frequencies of T allele were calculated for the case group and the control group, from the corresponding genotype distribution. The results were compared and disagreements were resolved by discussion.
Statistical analysis
Available data were entered into Cochrane Review Manager (RevMan, version 5, Oxford, UK) and analyzed. The pooled statistic was counted using the fixed effects model, but a random effects model was conducted when the p value of heterogeneity test was less than 0.1. Results were expressed with odds ratios (OR) for dichotomous data, and 95% confidence intervals (CI) were also calculated. p < 0.05 was required for the overall OR to be deemed statistically significant. I2 was used to test the heterogeneity between the included studies.
Results
Study characteristics
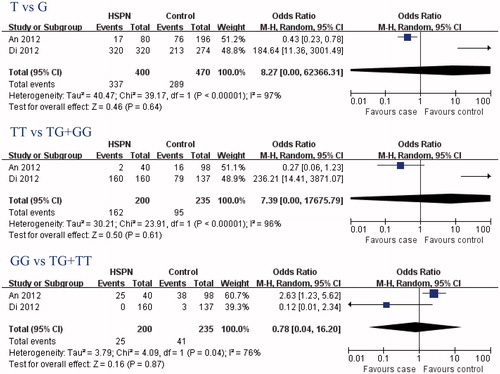
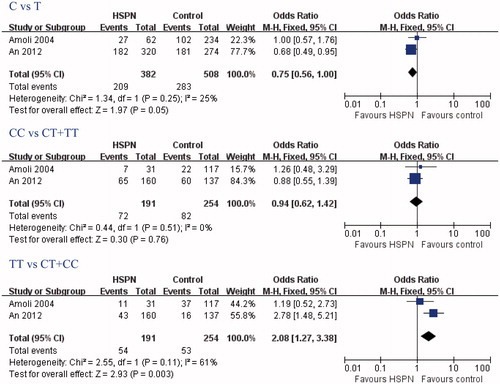
The search yielded 54 references, 6 from Pubmed, Embase, and Cochrane Library, 48 from CBM-disc. According to the inclusion and exclusion criteria, three articlesCitation4,Citation10,Citation11 were identified for the analysis of the between eNOS gene polymorphism and HSP or HSPN susceptibility in our review. Two studiesCitation4,Citation11 were conducted on the relationship between eNOS G894T gene polymorphism and HSPN susceptibility (), and two reportsCitation10,Citation11 were conducted on the relationship between eNOS T786C gene polymorphism and HSP susceptibility ().
Table 1. Characteristics of the studies evaluating the effects of eNOS G894T gene polymorphism on HSPN/HSP risk.
Table 2. Characteristics of the studies evaluating the effects of eNOS T786C gene polymorphism on HSPN/HSP risk.
Association of the eNOS G894T gene polymorphisms with HSPN/HSP risk, and the risk of HSP patients developing into HSPN
In this meta-analysis, eNOS G894T gene polymorphisms were not associated with HSPN risk (T-allele: OR = 8.27, 95% CI: 0.00–62366.31, p = 0.64; TT genotype: OR = 7.39, 95% CI: 0.00–17675.79, p = 0.61; GG genotype: OR = 0.78, 95% CI: 0.04–16.20, p = 0.87; and ) and the risk of HSP patients developing into HSPN (T allele: OR = 0.81, 95% CI: 0.43–1.52, p = 0.51; TT genotype: OR = 0.62, 95% CI: 0.12–3.15, p = 0.57; GG genotype: OR = 1.22, 95% CI: 0.57–2.62, p = 0.61; ). Interestingly, eNOS G894T T allele and GG genotype were associated with HSP susceptibility (T allele: OR = 0.53, 95% CI: 0.34–0.82, p = 0.005; GG genotype: OR = 2.16, 95% CI: 1.21–3.87, p = 0.01; ), but not the TT genotype (OR = 0.43, 95% CI: 0.17–1.11, p = 0.08; ).
Table 3. Meta analysis of the association of eNOS gene polymorphism with risk of HSPN/HSP risk.
Association of the eNOS T786C gene polymorphisms with HSPN/HSP risk, and the risk of HSP patients developing into HSPN
In this meta-analysis, eNOS T786C TT genotypes were associated with HSPN risk, but C allele and CC genotype were not (TT genotype: OR = 2.08, 95% CI: 1.27–3.38, p = 0.03; C allele: OR = 0.75, 95% CI: 0.56–1.00, p = 0.05; CC genotype: OR = 0.94, 95% CI: 0.62–1.42, p = 0.76; and ). eNOS T786C gene polymorphisms was not associated with HSP risk (C allele: OR = 0.89, 95% CI: 0.55–1.44, p = 0.64; CC genotype: OR = 1.11, 95% CI: 0.48–2.55, p = 0.81; TT genotype: OR = 1.37, 95% CI: 0.68–2.74, p = 0.37; ). Furthermore, eNOS T786C gene polymorphisms were also not associated with the risk of HSP patients developing into HSPN (C allele: OR = 1.36, 95% CI: 0.59–3.18, p = 0.47; CC genotype: OR = 1.46, 95% CI: 0.33–6.53, p = 0.62; TT genotype: OR = 0.69, 95% CI: 0.21–2.25, p = 0.54; ).
Discussion
The impairment of endothelial NO generation brought about by gene polymorphism is considered to be the major deterioration factor to be associated with the HSP/HSPN risk. There was rare genetic molecular marker to predict the onset of HSP/HSPN. This meta-analysis was performed to explore whether the eNOS gene polymorphism could predict the susceptibility of HSP/HSPN.
Henoch-Schönlein purpura is an immune complex-mediated disease predominantly characterized by the deposition of circulating immune complexes containing immunoglobulin A (IgA) on the walls of small vessels.Citation12 In this study, the relationship between eNOS gene polymorphism and the susceptibility of HSP was assessed. We found that eNOS G894T T allele and GG genotype were associated with HSP susceptibility, but not the TT genotype. Furthermore, eNOS T786C gene polymorphisms was not associated with HSP risk. However, the number of studies included was small, and the evidence was less robust. More studies should be performed in the future.
Henoch-Schönlein purpura is a form of systemic vasculitis that can progress to HSPN.Citation13 In this meta-analysis, the relationship between eNOS gene polymorphisms and HSPN risk was assessed. The results indicated that eNOS T786C TT genotype was associated with HSPN susceptibility, but C allele and CC genotype were not; and eNOS G894T gene polymorphism was not associated with HSPN susceptibility. Unfortunately, the number of studies included in this meta-analysis was small, and more studies should be conducted in future.
The prognosis of HSP is mostly dependent upon the severity of renal involvement.Citation14 In this meta-analysis, we also study the risk of progression of HSP into HSPN, and we found that eNOS G894T and T786C TT gene polymorphism were not associated with the risk of patients with HSP developing into HSPN. However, the number of studies included in this meta-analysis was small, and more studies should be conducted in future.
In our investigation, we found that eNOS G894T gene polymorphism was not associated with HSPN susceptibility and the risk of patients with HSP developing into HSPN was more. Interestingly, eNOS G894T T allele and GG genotype were associated with HSP susceptibility, but the TT genotype was not. eNOS T786C TT genotype was associated with HSPN susceptibility, but C allele and CC genotype were not. Furthermore, eNOS T786C gene polymorphism was not associated with HSP risk and the risk of patients with HSP developing into HSPN was more. These findings should be regarded cautiously because many other ingredients, such as heterogeneity of enrolled cases, limited statistical power, variable study designs and different interventions, were closely related to affect the results. Undoubtedly, the limitations mentioned above might affect our final conclusions.
In conclusion, the results in our study support that eNOS T786C TT genotype was associated with eNOS G894T T allele and GG genotype were associated with HSP susceptibility. However, more case-control association investigations on larger, stratified populations are required to further clarify the role of this eNOS gene polymorphism in HSP/HSPN susceptibility.
Declaration of interest
The authors declare no competing interests.
This study was supported by the sub-item of 985 Project Foundation of Sun Yat-Sen (The Hundred Talents Program Foundation; No. 88000-3311300).
References
- Qin YH, Zhou TB, Lei FY, et al. Cut-off values for serum matrix metalloproteinase-9: Is there a threshold to predict renal involvement for Henoch-Schonlein purpura in children? Nephrology (Carlton). 2011;16(1):93–99
- Zhou TB, Ou C, Qin YH, Luo W. A meta-analysis of the association between angiotensin-converting enzyme insertion/deletion gene polymorphism and Henoch-Schonlein purpura nephritis risk in Asian children. Clin Exp Rheumatol. 2012;30(2):315–316
- An J, Lu Q, Zhao H, Cao Y, Yan B, Ma Z. A study on the association between C1GALT1 polymorphisms and the risk of Henoch-Schonlein purpura in a Chinese population. Rheumatol Int. 2013;33(10):2539–2542
- Di B, Li X, Song L, Wang Q, Liu S. Association study of ACE and eNOS single nucleotide polymorphisms with Henoch-Schonlein purpura nephritis. Mol Med Rep. 2012;6(5):1171–1177
- Nalbantoglu S, Tabel Y, Mir S, Serdaroglu E, Berdeli A. Association between RAS gene polymorphisms (ACE I/D, AGT M235T) and Henoch-Schonlein purpura in a Turkish population. Dis Markers. 2013;34(1):23–32
- Wang JJ, Shi YP, Huang Y, Wu C, Li XC. Association of tumor necrosis factor-alpha gene polymorphisms with Henoch-Schonlein purpura nephritis in children. Zhongguo Dang Dai Er Ke Za Zhi. 2013;15(2):88–90
- Vecoli C. Endothelial nitric oxide synthase gene polymorphisms in cardiovascular disease. Vitam Horm. 2014;96:387–406
- Zhou TB, Xu HL, Yin SS. Association between endothelial nitric oxide synthase Glu298Asp gene polymorphism and diabetic nephropathy susceptibility. Ren Fail. 2013;35(1):173–178
- Zhou TB, Yin SS. Association of endothelial nitric oxide synthase Glu298Asp gene polymorphism with the risk of end-stage renal disease. Ren Fail. 2013;35(4):573–578
- Amoli MM, Garcia-Porrua C, Calvino MC, Ollier WE, Gonzalez-Gay MA. Lack of association between endothelial nitric oxide synthase polymorphisms and Henoch-Schonlein purpura. J Rheumatol. 2004;31(2):299–301
- An J, Yu J, Mei S, et al. Association of eNOS gene polymorphism with genetic susceptibility to Henoch-Schönlein purpura. J Mudanjiang Med Univ. 2012;33(1):1–3
- Cao N, Chen T, Guo ZP, Li MM, Jiao XY. Elevated serum levels of visfatin in patients with Henoch-Schonlein purpura. Ann Dermatol. 2014;26(3):303–307
- Ren P, Han F, Chen L, Xu Y, Wang Y, Chen J. The combination of mycophenolate mofetil with corticosteroids induces remission of Henoch-Schonlein purpura nephritis. Am J Nephrol. 2012;36(3):271–277
- Davin JC, Coppo R. Henoch-Schonlein purpura nephritis in children. Nat Rev Nephrol. 2014;10:563–573