Abstract
Loss of peritoneal function due to peritoneal fibrosing syndrome (PFS) is a major factor leading to treatment failure in chronic peritoneal dialysis (PD) patients. Although the precise biologic mechanisms responsible for these changes have not been defined, the general assumption is that alterations in peritoneal function are related to structural changes in the peritoneal membrane. Studies of the peritoneal membrane by non-invasive ultrasonography (US) in chronic PD patients are limited. The aim of the present study is to assess the relationship between functional parameters of peritoneum and peritoneal thickness measured by US in children treated by chronic PD. We recruited two groups of patients: 23 subjects (13 females, 10 males) on chronic PD (patient group) and 26 (7 females, 19 males) on predialysis out-patient follow-up (creatinine clearance: 20–60 mL/min/1.73 m2) (control group). Age, sex, weight, height, body mass index (BMI), chronic PD duration, episodes of peritonitis and the results of peritoneal equilibration test (PET) were recorded. Hemoglobin (Hb), blood pressure (BP), left ventricular mass index (LVMI) and renal osteodystrophy (ROD) parameters were also obtained. The thickness of the parietal peritoneum was measured by trans-abdominal US in all children. Statistical analyses were performed by using Student's t and Pearson's correlation tests. Mean peritoneal thickness in chronic PD patients (1028.26 ± 157.26 μm) was significantly higher than control patients (786.52 ± 132.33). Mean peritoneal thickness was significantly correlated with mean body height (R2 = 0.93, p < 0.05), BMI (R2 = 0.25, p < 0.05), chronic PD duration (R2 = 0.64, p < 0.05), episodes of peritonitis (R2 = 0.93, p < 0.05), D/Pcreatinine (R2 = 0.76, p < 0.05) and D4/D0glucose (R2 = 0.81, p < 0.05). No correlation was found between peritoneal thickness and Hb, BP, LVMI and ROD parameters. In conclusion, ultrasonographic measurement of peritoneal membrane thickness is a simple and non-invasive method in chronic PD children. This diagnostic tool likely enables to assess peritoneal structure and function in these patients.
Introduction
Peritoneal dialysis (PD) is the preferred renal replacement treatment in children with end-stage renal disease (ESRD).Citation1–3 However, interstitial fibrosis and hyalinization of vessels of peritoneal membrane due to ultrafiltration failure and solute transport problems have resulted in the need for termination of PD over time. In USA, more than half of the chronic PD patients experienced peritoneal membrane failure due to peritoneal fibrosing syndrome (PFS) after a 5-year of PD treatment period.Citation1 In addition, drop out from PD due to PFS was reported 16% within the first two years of PD treatment.Citation4 On the other hand, Williams et al.Citation2 showed high rates of PFS (61%) in peritoneal biopsy specimens in patients after the termination of PD.
Although the definitive diagnosis of PFS can be made by peritoneal biopsy, ethical issues, difficulty of the process and insufficient knowledge of PFS are the major obstacles for routine clinical application of peritoneal membrane biopsy.Citation3,Citation5–10 Measurement of cytokines levels which are responsible in PFS progression can be an alternative diagnostic tool.Citation3 However, there is no consensus on the time and the frequency of cytokines monitoring in the diagnosis of PFS. It is also unclear whether monitoring these cytokines is helpful.Citation11 The measurement of peritoneal membrane permeability is of utmost importance to determine the ultrafiltration failure and solute transport problems resulting from interstitial fibrosis and hyalinization of peritoneal membrane vessels. However, the measurement of peritoneal membrane permeability shows only the instant peritoneal status, not the progression.Citation12–14 On the other hand, because it is a non-invasive technique and easy to perform, peritoneal membrane thickness measurements by ultrasonography (US) can be an alternative method to determine PFS. To date, there is limited data regarding the measurement of peritoneal membrane thickness for predicting the PFS and, hence, peritoneal membrane dysfunction in children on chronic PD.Citation15–19 Periodic monitoring of changes in peritoneal membrane thickness assessed by US will make the patient management less likely that treatment complications, during the follow-up, will occur.
The aim of this study was to determine the value of ultrasonographic measurement of peritoneal membrane thickness for the diagnosis of PFS in pediatric chronic PD patients.
Materials and methods
Population
This single-center, cross-sectional study was conducted on 23 children (13 females, 10 males) (study group) with ESRD treated by chronic PD, at the Department of Pediatric Nephrology, Izmir Tepecik Training and Research Hospital, Turkey. The control group consisted of 26 (19 males, 7 females) age and sex matched the chronic kidney disease patients with a creatinine clearance (CrCl) of 20–60 mL/min/1.73 m2 who were under follow-up at the out-patient clinic of our unit.
Age, gender, height and weight were recorded for each patient. The body mass index (BMI) was also calculated. Body height (H) and weight (W) were expressed as HZ score (=observed height − median height/standard deviation) and WZ score (=observed weight − median weight/standard deviation), respectively. Blood pressure and left ventricular mass index (LVMI) values as well as serum hemoglobin, albumin, calcium, phosphorus, calcium–phosphorus product and parathyroid hormone (PTH) levels were also obtained. These parameters were also used as markers of dialysis adequacy in PD patient group. In chronic PD patient group; residual renal function, weekly CrCl, Kt/V values, peritoneal equilibrium test (PET) scores and peritonitis frequency as well as underlying etiology, diagnosis age, dialysis duration, type of PD and PD prescription were also recorded. Peritoneal membrane thickness measurement was done in both the study group and controls.
Peritoneal membrane thickness measurement
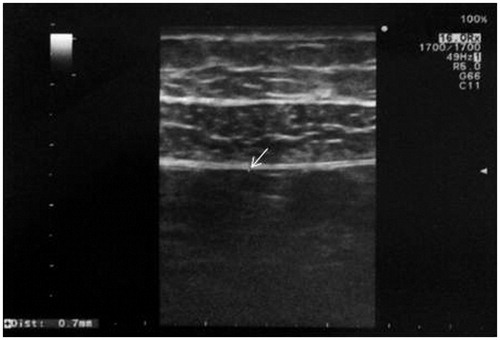
All measurements were taken using a 13–5 MHz linear transducer (high-resolution probe) and a Siemens Antares (Siemens AG, Erlangen, Germany) US unit. The ultrasonographic measurements were obtained on each of the abdominal quadrants while the patient was in the supine position. The probe was held perpendicular to both the midclavicular line and the body surface. Measurement of the parietal peritoneum, defined as the inner hyperechogenic line surrounding the abdominal cavity, was obtained on each abdominal quadrant in each patient at the level of the midclavicular longitudinal line (). The mean of the four measurements was calculated for each patient. In cases where the peritoneal thickness in one of the lower quadrants could not be obtained because of the presence of the peritoneal catheter, the mean of the remaining three values was calculated. Peritoneal membrane thickness was expressed in microns.
Statistics
Statistical analyses were made by using IBM SPSS 16.0 software (SPSS, Chicago, IL). The mean differences between groups were compared using the ANOVA and Student's t-tests. Correlations between data sets were evaluated using Pearson's correlation test. Effectors of peritoneal membrane thickness were tested by multiple regression analyses. Pearson's correlation coefficient and descriptive coefficient were given as a “r” and “R2”, respectively. Results were expressed as the mean ± SD. A p value of less than 0.05 was considered to be significant.
Results
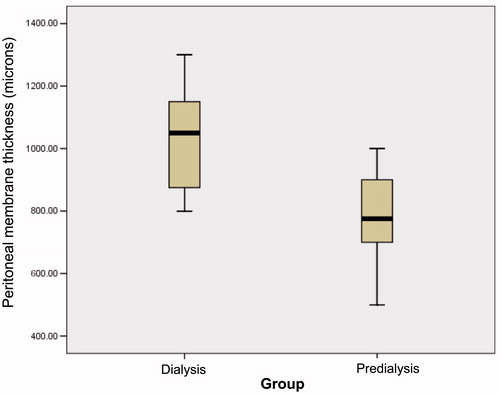
Underlying diseases of the patients are given in . Urological problems (vesicoureteral reflux, chronic pyelonephritis, obstructive uropathy and neurogenic bladder) were by far the most common pathologies (65.4%) in the study group. Demographic and physical features of the patients are shown in . There were no significant differences between study group and controls with respect to age, gender, growth and disease duration (p > 0.05). The LVMI and PTH levels were higher in PD group than in control group (p < 0.05) whereas hemoglobin and albumin levels were significantly higher in controls (p < 0.05). Peritoneal wall thickness was found to be higher in PD group (p = 0.01) (). Peritoneal membrane thickness was significantly correlated with patient height (PD group R2 = 0.939, controls R2 = 0.965) () and BMI (PD group R2 = 0.252, controls R2 = 0.206) (). Peritoneal thickness showed a positive linear correlation with dialysis duration (R2 = 0.644) (), D/PCreatinine (R2 = 0.764) () and peritonitis frequency (R2 = 0.933) () whereas negative linear correlation with D4/D0Glucose (R2 = 0.819) (). No correlation was found with Kt/V (R2 = 0.0003), LVMI (R2 = 0.025), blood pressure (systolic R2 = 0.01, diastolic R2 = 0.022), hemoglobin concentration (R2 = 0.064), CaxP product (R2 = 0.028) and PTH (R2 = 0.069) levels.
Figure 3. The relationship between peritoneal wall thickness and body height (A) and body mass index (B).
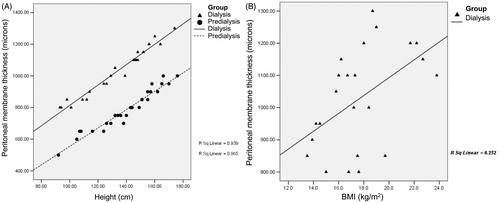
Figure 4. The relationship between peritoneal wall thickness and dialysis duration (A), D/PCreatinine (B), episodes of peritonitis (C) and D4/D0Glucose (D).
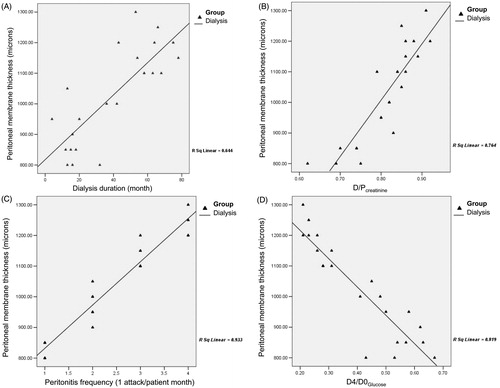
Table 1. Underlying diseases of the patients.
Table 2. Demographic, physical and laboratory features of the patients.
Discussion
It is well known that clinical, biochemical and kinetic parameters have been used to evaluate peritoneal dialysis adequacy in PD patients. However, these parameters alone are not enough to determine PD efficiency. On the other hand, the assessment of peritoneal membrane morphology by biopsy is another marker in evaluating PD adequacy, peritoneal biopsy is an invasive method and difficult to harvest it repeatedly. Therefore, ultrasonographic measurement of peritoneal membrane thickness seems logical and reasonable, because it is easy to perform as well as a non-invasive and a repeatable tool. This approach could be an alternate method for the detection of peritoneal anatomy, allowing us to predict the functional properties of peritoneal membrane in PD patients. We believe that, ultrasonographic evaluation of peritoneal membrane in conjunction with other PD adequacy markers would have favorable effects on optimizing the PD prescription, patient outcome and also minimize treatment drop-out.
Morphologically and functionally stable peritoneal membrane is mandatory for longevity and adequacy of PD. It has been reported that membrane failure is the most important reason for PD drop-out.Citation20 Longer exposure of non-biocompatible dialysis solutions and frequent peritonitis episodes are the major causes of functional deterioration of the peritoneal membrane.Citation2,Citation10,Citation21–23 Loss of peritoneal membrane function yields a close relationship with the degree of mesothelial degeneration, submesothelial thickness, fibrosis and sclerosis in peritoneal membrane.Citation2,Citation24 In their biopsy-specimen-based study, Williams et al.Citation2 found an increased peritoneal membrane in PD patients. They suggested that peritoneal biopsy is a gold standard method for assessment of peritoneal membrane morphology. However, it has also been reported that this invasive method is not suitable for routine clinical assessment.Citation15–19 It has been reported that ultrasonographic determination of peritoneal wall thickness was a simple, non-invasive and repeatable technique to measure peritoneal changes.Citation15–19 The implementation of sonographic peritoneal measurement together with PD adequacy tests provides useful information about peritoneal membrane function and morphology.Citation15,Citation17–19 We believe that ultrasonographic imaging of peritoneum is a powerful technique to predict peritoneal function and it should be part of the routine assessment of PD patients.
There is a strong relationship between functional deterioration of the peritoneal membrane and PD duration. It has been reported that morphological changes in peritoneal membrane were associated with time on PD treatment.Citation2,Citation10,Citation21,Citation22 Several studies showed a positive correlation between PD duration and peritoneal wall thickness.Citation15,Citation17–19 In our study, peritoneal membrane thickness increased significantly (R2 = 0.644, ) with the duration of PD treatment. This finding supports that peritoneal membrane thickening increases with length of time on PD, which reflects the peritoneal functional changes.
Peritoneal equilibrium test studies have been applied to manipulate dialysis prescriptions in chronic PD patients.Citation12,Citation25 In our series, we also compared peritoneal membrane thickness measured by US with PET results. Lee et alCitation15 showed an association between peritoneal wall thickness and D/Pcreatinine. Similarly, Duman et alCitation17 observed a positive correlation between peritoneal wall thickness and D/Pcreatinine, whereas a negative correlation with D4/D0glucose. In subsequent years, other published reports of peritoneal thickness by ultrasonography supported these findings.Citation18,Citation19 In agreement with those findings, in our study, peritoneal membrane thickness showed a positive correlation with D/Pcreatinine (R2 = 0.764, ) and negative correlation with D4/D0Glucose (R2 = 0.819, ). These results support that serial measurement of peritoneal wall thickness by US could reflect the sequential changes in peritoneal membrane structure and hence, PD adequacy. On the other hand, our patients were attained the Kt/V targets (mean: 2.96 ± 0.94) recommended by the 2006 K/DOQI work group.Citation26 However, we did not find any significant relationship between peritoneal wall thickness and Kt/V values. A possible explanation for this reason might be the smaller number of patients in our series.
Peritonitis is an important and feared complication of PD leading to accelerated peritoneal fibrosis process due to acute inflammation and locally releasing cytokines (e.g., transforming growth factor, fibroblast growth factor).Citation5,Citation7,Citation8,Citation15–17,Citation27,Citation28 Previous studies have found no significant association between peritoneal wall thickness by US and peritonitis frequency.Citation15,Citation17–19 On the contrary, however, we found for the first time, a positive correlation between peritoneal thickness and episode of peritonitis (R2 = 0.933, ).
We also examined the relationship between clinical and laboratory parameters with peritoneal membrane thickness in our patients. In the analysis of the association between clinical and laboratory parameters with peritoneal thickness by US, only the anthropometric indices (weight, height and BMI) showed significant association with peritoneal thickness.
Our study has certain limitations, such as the low number of patients and the cross-sectional, single-center design, which limits the generalizability of the findings. Also, the lack of long-term and sequential measurements of peritoneal membrane thickness in our series raises the question of whether peritoneal thickness by US allows us to determine peritoneal function and PD survival. Finally, we were not able to perform peritoneal membrane biopsy; an ultrasound-pathology correlation study would be useful to show the predictive value of peritoneal membrane thickness by US for peritoneal membrane function.
In conclusion, peritoneal membrane fibrosis is unavoidable in long-term PD patients. Ultrasonographic measurement of peritoneal membrane thickness is a simple and non-invasive method in chronic PD patients. This diagnostic tool likely enables to assess peritoneal membrane structure and function in these patients. Therefore, early evaluation of peritoneal membrane characteristics by means of US can improve patient outcomes.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Davies SJ, Phillips L, Griffiths AM, Russell RH, Naish PF, Russell GI. What really happens to people on long-term peritoneal dialysis? Kidney Int. 1998;54:2207–2217
- Williams JD, Craig KJ, Topley N, et al. Morphologic changes in the peritoneal membrane of patients with renal failure. J Am Soc Nephrol. 2002;13:470–479
- Hung KY, Huang JW, Tsai TJ, Hsieh BS. Peritoneal fibrosing syndrome: Pathogenetic mechanism and current therapeutic strategies. J Chin Med Assoc. 2005;68:401–405
- Hung KY, Lin TJ, Tsai TJ, Chen WY. Impact of peritoneal membrane transport on technique failure and patient survival in a population on automated peritoneal dialysis. ASAIO J. 1999;45:568–573
- Hung KY, Shyu RS, Fang CC, Lee PH, Tsai TJ, Hsieh BS. Dipyridamole inhibits human peritoneal mesothelial cell proliferation in vitro and attenuates rat peritoneal fibrosis in vivo. Kidney Int. 2001;59:2316–2324
- Hung KY, Shyu RS, Huang JW, Tsai TJ, Chen WY. Natural changes in peritoneal equilibrium test results in CAPD patients: A retrospective, seven-year cohort survey. Artif Organs. 2000;24:261–264
- Stoenoiu MS, De Vrises AS, Brouet A, et al. Experimental diabetes induces functional and structural changes in the peritoneum. Kidney Int. 2002;62:668–678
- Williams JD, Craig KJ, Topley N, Williams GT. Peritoneal dialysis: Changes to the structure of the peritoneal membrane and potential for biocompatible solutions. Kidney Int. 2003;63:S158–S161
- Margetts PJ, Gyorffy S, Kolb M, et al. Antiangiogenic and antifibrotic gene therapy in chronic infusion model of peritoneal dialysis in rats. J Am Soc Nephrol. 2002;13:721–732
- Honda K, Hamada C, Nakayama M, et al; Peritoneal Biopsy Study Group of the Japanese Society for Peritoneal Dialysis. Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: A quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol. 2008;3:720–728
- Ebinc FA, Derici U, Gonen S, et al. TGF-beta-1 gene polymorphisms and peritoneal equilibration test results in CAPD patients. Ren Fail. 2008;30:15–19
- Twardowski ZJ. Pathophysiology of peritoneal transport. Contrib Nephrol. 2006;150:13–19
- Twardowski ZJ. Clinical value of standardized equilibration tests in CAPD patients. Blood Purif. 1989;7:95–108
- Rodby RA, Firanek CA, Sarpolis AL. Re-evaluation of solute transport groups using the peritoneal equilibration test. Perit Dial Int. 1999;19:438–441
- Lee TC, Yang JY, Wang HP, Tsai TJ, Yang Y. Peritoneal thickening is not inevitable in long-term peritoneal dialysis and is associated with peritoneal transport characteristics: A two-centre sonographic study. Nephrol Dial Transplant. 2008;23:1005–1010
- Faller U, Stegen P, Klaus G, Mehls O, Tröger J. Sonographic determination of the thickness of the peritoneum in healthy children and paediatric patients on CAPD. Nephrol Dial Transplant. 1998;13:3172–3177
- Duman S, Ozbek SS, Gunay ES, et al. What does peritoneal thickness in peritoneal dialysis patients tell us? Adv Perit Dial. 2007;23:28–33
- Temiz G, Sahin G, Mukerrem G, et al. Ultrasonographic evaluation of peritoneal membrane thickness and comparison with the effectiveness and duration of CAPD. Int Urol Nephrol. 2013;45:1761–1766
- Caltik A, Akyüz SG, Bülbül M, et al. Can sonographic peritoneal thickness be used to follow pediatric patients on peritoneal dialysis? Pediatr Nephrol. 2013;28:811–817
- Coles GA, Williams JD, Topley N. Peritoneal inflammation and long-term changes in peritoneal structure and function. In: Gokal R, Khanna R, Krediet RT, Nolph KD, eds. Textbook of Peritoneal Dialysis. 2nd ed. Dordrecht: Kluwer Academic Publishers; 2000:566–583
- Plum J, Hermann S, Fusshöller A, et al. Peritoneal sclerosis in peritoneal dialysis patients related to dialysis settings and peritoneal transport properties. Kidney Int. 2001;78:S42–S47
- Margetts PJ, Bonniaud P. Basic mechanisms and clinical implications of peritoneal fibrosis. Perit Dial Int. 2003;23:530–541
- Davies SJ, Bryan J, Phillips L, Russell GI. Longitudinal changes in peritoneal kinetics: The effects of peritoneal dialysis and peritonitis. Nephrol Dial Transplant. 1996;11:498–506
- Flessner MF, Choi J, Vanpelt H, et al. Correlating structure with solute and water transport in a chronic model of peritoneal inflammation. Am J Renal Physiol. 2006;290:232–240
- Twardowski ZJ, Nolph KD, Khanna R, et al. Peritoneal equilibration test. Perit Dial Bull. 1987;7:138–147
- National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 Updates: Hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis. 2006;48:S1–S322
- Topley N, Liberek T, Davenport A, Li FK, Fear H, Williams JD. Activation of inflammation and leukocyte recruitment into the peritoneal cavity. Kidney Int. 1996;56:S17–S21
- Mlambo NC, Hylander B, Brauner A. Increased levels of transforming growth factor beta 1 and basic fibroblast growth factor in patients on CAPD: A study during non-infected steady state and peritonitis. Inflammation. 1999;23:131–139