Abstract
Background: This was controversial whether vitamin E-coated dialyzer therapy was beneficial for the complications associated with hemodialysis. Therefore, we performed this systematic review to evaluate the effects of vitamin E-coated dialyzer. Methods: Related trials were searched from multiple electronic databases. We conducted meta-analysis to assess changes in the predefined outcomes using RevMan 5.3 software. Results: Meta-analysis showed vitamin E-coated dialyzer therapy could decrease erythropoietin (EPO) resistance index (SMD, −0.24; 95% CI, −0.47 to −0.01; p = 0.04). However, pooled-analysis showed vitamin E-coated dialyzer therapy could not decrease weekly EPO dose (SMD, −0.11; 95% CI, −0.32 to 0.09; p = 0.28) and intima–media thickness (IMT) of the carotid artery (MD, −0.09; 95% CI, −0.2 to 0.01; p = 0.09), and vitamin E-coated dialyzer therapy did not improve the serum hemoglobin (MD, −0.03; 95% CI, −0.18 to 0.13; p = 0.74), albumin levels (SMD, −0.64; 95% CI, −1.62 to 0.34; p = 0.2), in addition, there was no significant difference in serum cholesterol (SMD, −0.07; 95% CI, −0.45 to 0.31; p = 0.71), triglycerides (MD, −2.77; 95% CI, −32.42 to 26.87; p = 0.85), high density lipoprotein (HDL) (SMD, 0.24; 95% CI, −0.14 to 0.62; p = 0.22) and low density lipoprotein (LDL) (SMD, 0.00; 95% CI, −0.38 to 0.37; p = 0.98) levels. Conclusions: Vitamin E-coated dialyzer may reduce the EPO resistance, but there is no conclusive evidence that vitamin E-coated dialyzer can improve the renal anemia, malnutrition, dyslipidemia and atherosclerosis status in hemodialysis (HD) patients. However, high-quality trials with hard clinical endpoints are required to fully elucidate the clinical value of vitamin E-coated dialyzer therapy.
Introduction
A status of excessive oxidative stress is highly prevalent in patients undergoing maintenance hemodialysis (HD), in part because of an increased free radicals production due to the interaction of blood with the dialyzer membranes during extracorporeal circulation,Citation1 and net losses of many soluble antioxidants.Citation2 The reactive oxygen species (ROS) can impair neighboring cells and evoke an inflammatory response,Citation3 which is increased in HD patients not only because of their uremic status per se, but also due to HD-related factors such as the chemical and biological quality of the dialysate, and the bio-incompatibility of dialysis membranes.Citation4 In addition, the circulating biomarkers of oxidative stress and chronic inflammatory cytokines such as malondialdehyde (MDA), C-reactive protein (CRP) or interleukin-6 (IL-6) are significantly elevated in maintenance HD patients.Citation5,Citation6 There is increasing evidence that increased oxidative stress and inflammatory cytokines play a key role in the pathogenesis of malnutrition,Citation7 resistance to erythropoietin,Citation8 dyslipidemiaCitation9 and atherosclerosisCitation10 related with hemodialysis.
Consequently, all these observations suggest that the direct scavenging of oxygen free radicals through the use of antioxidants (e.g., vitamin E) may be an attractive therapeutic strategy for anemia or malnutrition in HD patients. This can be achieved by taking oral vitamin E supplementation or specifically binding vitamin E to the dialysis membrane site. Recently two system reviews have concluded that vitamin E-coated dialyzer treatment could reduce the oxidative stress and inflammatory status in HD patients.Citation11,Citation12 In this regard, some encouraging results have been obtained with vitamin E-modified membrane dialyzer could improve renal anemia, erythropoietin (EPO) consumption and atherosclerosis.Citation8,Citation13,Citation14 However, there was controversy about these effects of vitamin E-coated dialyzer.Citation15,Citation16. Therefore, we undertook the present meta-analysis mainly to reconcile the various strands within the dispute about the effects of vitamin E-coated dialyzer on renal anemia, resistance to EPO, malnutrition, dyslipidemia and atherosclerosis in HD patients.
Materials and methods
Inclusion criteria
Types of studies: Published reports of randomized controlled trials (RCTs) and quasi-RCTs comparing vitamin E-coated HD membranes with conventional unmodified dialyzer (polysulfone or cellulose and so on) in English with available data for our prescribed outcomes.
Type of participants: The studies were restricted to any patients with end stage renal disease (ESRD) requiring HD therapy stability.
Type of interventions: Studies comparing conventional dialyzer (unmodified cellulose-derived membranes or synthetic membranes dialyzers) with vitamin E-coated dialyzer for chronic HD patients.
Type of outcome measures: Anemia parameters: hemoglobin (HB), weekly EPO dose, EPO resistance index; nutrition parameters: serum albumin (Alb); lipid parameters: serum triglycerides, cholesterol, low density lipoprotein (LDL) and high density lipoprotein (HDL) levels; atherosclerosis parameters: the intima–media thickness (IMT) of the carotid artery; dialysis adequacy: fractional clearance of urea (Kt/V).
Data sources and search strategy
We searched the medical literature for clinical studies examining the effect of vitamin E-coated dialyzer. Four English electronic databases were initially searched: SCOPUS, MEDLINE, EMBASE and Cochrane Central Register of Controlled Trials (CCRCT) (all to October 2014). Article search terms included: hemodialysis, dialysis, renal replacement therapy, dialyzer, vitamins, vitamin E, tocopherol, alpha-tocopherol, beta-tocopherol, anemia, nutrition, lipid, atherosclerosis, hemoglobin, erythropoietin, erythropoietin resistance index, albumin, cholesterol, triglyceride, HDL, LDL, IMT, etc. In order to identify further potentially relevant studies, we also searched various network databases such as Google Scholar (http://scholar.google.com/), and the reference lists of included studies were manually checked.
Study selection and data extraction
Two authors (B.Y. and J.H.) independently screened the titles and abstracts of identified studies. Only parallel RCTs or quasi-RCTs and the first period of crossover RCTs or quasi-RCTs on examining the effects of vitamin E-coated dialyzer on markers of anemia, nutrition, lipid, atherosclerosis were included. The full-text articles were retrieved for comprehensive review and rescreened independently. Data extraction was performed by two authors (B.Y. and J.H.) using the standard data extraction forms.
Study quality assessment
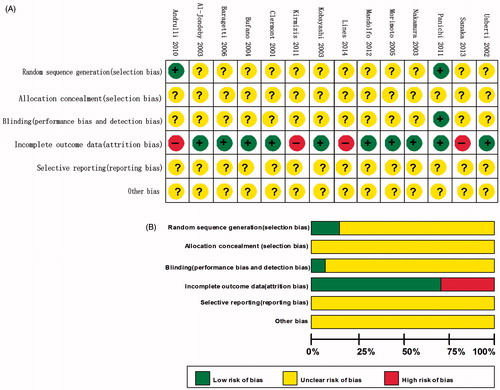
The method quality of each study was evaluated by two authors (J.H. and A.M.L.) independently using the Cochrane risk of bias tool,Citation17 this included six evaluation criterias: random sequence generation (selection bias), allocation sequence concealment (selection bias), blinding (performance and detection bias), selective outcome reporting (reporting bias), incomplete outcome data (attrition bias) and other potential sources of bias. The judgment for each criterion was indicating as “low risk of bias”, “high risk of biases”, or “unclear risk of biases”.
Data synthesis and statistical analysis
The extracted outcome from studies was continuous data, so treatment effects were summarized using the mean difference (MD) and 95% confidence interval (CI) was also calculated. As different units of some parameters (e.g., weekly EPO dose, EPO resistance index, serum Alb, cholesterol levels) were used in different trials, so we used standardized mean (SMD) to report, the Cochrane Review Manager (RevMan 5.3, Cochrane Collaboration, Oxford, UK) software was used for all the pooled analyses.Citation18
Assessment of heterogeneity and subgroup analysis
Existence of statistical heterogeneity among effect sizes was analyzed using the Q statistic (p < 0.1 indicating significant) and the I2 statistic (I2 value of >50% correspond to high heterogeneity).Citation19 If heterogeneity was not present, we performed a fixed-effect meta-analysis to assess net changes in the same outcome variables. If there was significant statistical heterogeneity, the random-effects model was performed. Subgroup analyses were conducted to explore potential sources of heterogeneity according to the controlled dialyzer type (biocompatible membranes dialyzer and bio-incompatible membranes dialyzer) and study duration (<6 months, 6 months and >6 months). Funnel plots were used to assess publication bias.Citation20
Results
Selection and identification of studies
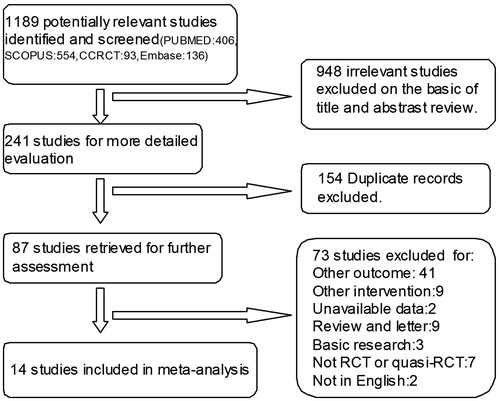
Our electronic search yielded 1189 studies, of which 948 studies were irrelevant to the study question. The remaining 241 articles were retrieved for detailed evaluation. Among them, duplicate records, non-RCTs or quasi-RCTs, review and letter, article not in English, other intervention and concerning other outcome were excluded. After the progressive selection process, 14 eligibility studiesCitation1,Citation8,Citation13–16,Citation21–28 including five crossover and nine parallel-arm trials were included in this meta-analysis ().
Characteristics of studies
The main characteristics of included studies are summarized in . The studies varied in sample size (16–305 patients). These 14 studies enrolled a total of 975 HD patients, among them, 473 were treated with vitamin E-coated dialyzer and 502 were treated with conventional dialyzer. Of these 14 included studies, seven of the 14 included studies compared vitamin E-coated dialyzer with polysulfone dialyzerCitation8,Citation14–16,Citation22,Citation25,Citation28 while four studies compared with cellulose membrane dialyzer.Citation1,Citation13,Citation21,Citation26 Mean age of study participants ranged from 51.9 to 72.3 years, with follow-up ranged from 1 to 12 months.
Table 1. Characteristic of included studies.
Quality assessment of included studies
Allocation: Randomization was unclear in 12 studies. Some randomized methods such as computer-generated randomization table and drawing random numbers were applied in two studies.Citation8,Citation22 None of the included trials clearly described the allocation concealment ways.
Blinding: Only one study performed by Panichi et al. was single blinded,Citation8 while other studies were not blinded designed.
Incomplete outcome data: Four studies reported withdrawals,Citation15,Citation16,Citation22,Citation24 but the results were not analyzed on an intention-to-treat basis. Ten trials were assessed to be low risk of attrition bias.
Selective reporting: None of the 14 included studies reported all expected outcomes.
Other potential sources of bias: Other potential bias was unable to be assessed due to insufficient information in the included trials. The summaries of the risk of bias findings are shown in .
Effects on renal anemia
The effect of vitamin E-coated dialyzer on HB level was assessed in six trials,Citation8,Citation14,Citation15,Citation21,Citation23,Citation25 this meta-analysis showed a nonsignificant increase in HB level compared with that of control (MD, −0.03; 95% CI, −0.18 to 0.13; p = 0.74; ), with significant heterogeneity between studies (p = 0.06; I2 = 50%). Six studies reported the EPO dose as mean and standard deviation,Citation8,Citation13–15,Citation23,Citation25 the fixed model was performed for meta-analysis. The pooled analysis showed that vitamin E-coated dialyzer therapy did not result in a significant decrease in EPO dose (SMD, −0.11; 95% CI, −0.32 to 0.09; p = 0.28, ). In the study performed by Andrulli et al.,Citation22 HB and weekly EPO dose were reported as median and range, in line with our pooled analysis, the result showed that vitamin E-coated dialyzer was not associated with a significant improvement in HB level and weekly EPO dose (p = 0.859, p = 0.362 respectively).Citation22
Figure 3. Forest plot of studies comparing the effect of vitamin E-coated dialyzer versus conventional dialyzer on HB level in hemodialysis patients.
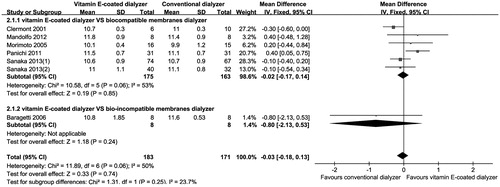
Figure 4. Forest plot of studies comparing the effect of vitamin E-coated dialyzer versus conventional dialyzer on EPO dose and EPO resistance index in hemodialysis patients.
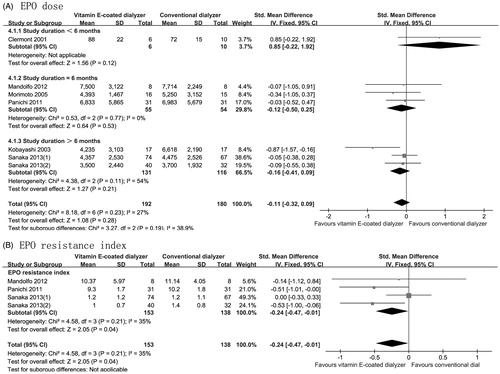
Three studies reported on EPO resistance index (n = 291), and the pooled analyzed were performed under a fixed-effects mode,Citation8,Citation14,Citation15 the analysis showed that vitamin E-coated dialyzer therapy resulted in a significant reduction in EPO resistance index (SMD, −0.24; 95% CI, −0.47 to −0.01; p = 0.04; ), with acceptable heterogeneity (p = 0.21; I2 = 35%). In the study performed by Lines et al.,Citation16 EPO resistance index data was reported as median and range, in contrast with our pooled analysis, the result showed that vitamin E-coated dialyzer was not associated with a significant decrease in EPO resistance index at the end of 12 months’ treatment (p = 0.2).Citation16
Effects on nutrition markers
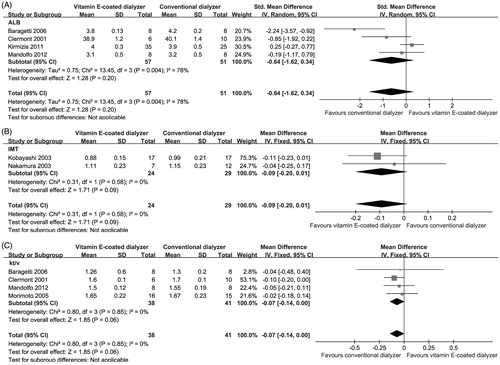
As shown in , the changes in serum Alb were evaluated in four trials (n = 108).Citation14,Citation21,Citation23,Citation24 When the meta-analysis was performed, no significant effect was found after conversion from conventional dialyzer to vitamin E-coated dialyzer (SMD, −0.64; 95% CI, −1.62 to 0.34; p = 0.2), with evidence of heterogeneity (p = 0.004; I2 = 78%).
Effects on serum lipid parameters
As seen in , serum cholesterol was compared between patients receiving vitamin E-coated dialyzer and those receiving conventional dialyzer in three trials involving 111 patients.Citation1,Citation24,Citation26 The meta-analysis showed that there was no statistically significant change in serum cholesterol in the vitamin E-coated dialyzer groups (SMD, −0.07; 95% CI, −0.45 to 0.31; p = 0.71), with minimal heterogeneity (p = 0.46; I2 = 0%).
Figure 6. Forest plot of studies comparing the effect of vitamin E-coated dialyzer versus conventional dialyzer on lipid parameters in hemodialysis patients.
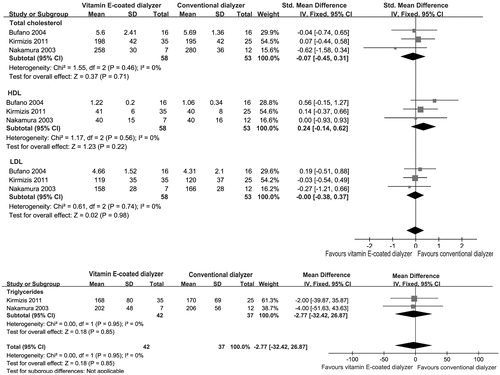
The changes in serum triglycerides fractions were analyzed from two trials.Citation24,Citation26 Again, there was no significant change of triglycerides in the vitamin E-coated dialyzer groups (MD, −2.77; 95% CI, −32.42 to 26.87; p = 0.85; ). In addition, the pooled analysis showed that vitamin E-coated dialyzer therapy could not change serum HDL and LDL levels (SMD, 0.24; 95% CI, −0.14 to 0.62, p = 0.22; SMD, −0.00; 95% CI, −0.38 to 0.37, p = 0.98, respectively).
Effects on atherosclerosis
It has been suggested that vitamin E-coated dialyzer may exert its favorable therapeutic effects on atherosclerosis. Two studies involving 53 patients were analyzed under a fixed model.Citation13,Citation26 However, no significant effect on IMT of the carotid artery was found in the vitamin E-coated dialyzer groups (MD, −0.09; 95% CI, −0.2 to 0.01; p = 0.09; ).
Effects on dialysis adequacy
Four studies involving 79 patients reported fractional clearance of urea (Kt/V) index as a measure of dialysis adequacy. In line with the research performed by Yang et al.,Citation12 our pooled analysis result showed that conversion from vitamin E-coated dialyzer to conventional dialyzer did not result in increase in Kt/V index (MD, −0.07; 95% CI, −0.14 to 0.00; p = 0.06), with minimal heterogeneity (p = 0.85; I2 = 0%; ).
Investigations of heterogeneity
We performed subgroup analysis to explore mean change in HB and weekly EPO dose in trials, stratified by dialyzer membranes biocompatibility and study duration. In brief, there was no significant effect on HB level using different biocompatible membranes dialyzer, but the use of vitamin E-coated dialyzer resulted in a significant decrease in weekly EPO dose compared with studies using bio-incompatible membranes (cellulose membrane) dialyzer (MD, −0.87; 95% CI, −1.57 to −0.16; p = 0.02).Citation13 In addition, use of vitamin E-coated dialyzer therapy resulted in a significant increase in HB level in trials of 6 months duration (MD, 0.36; 95% CI, 0.07 to 0.65; p = 0.01),Citation8,Citation14,Citation25 while there was no effect on HB level in those of >6 months duration (MD, −0.12; 95% CI, −0.37 to 0.12; p = 0.32).Citation15,Citation21 We used funnel plots to assess the publication bias, as shown in , most funnel plots of the outcomes such as EPO dose, HB, TG, HDL, LDL were symmetric. However, there were some key outcomes such as Alb and Kt/V was asymmetric, which suggested that there might be publication bias among these studies.
Table 2. Summary effect of vitamin E-coated dialyzer on maintenance hemodialysis patients.
Discussion
The main finding based on this system review is that vitamin E-coated dialyzer may reduce the EPO resistance, but there is no conclusive evidence that vitamin E-coated dialyzer can improve the renal anemia, malnutrition and atherosclerosis status in HD patients. As these there is no specific defined hypothesis before this exploratory meta-analysis. The pooled analysis results can only suggest vitamin E-coated dialyzer therapy effects but dose not demonstrate them.
As a consequence of the low biocompatibility of conventional hemodialysis membrane dialyzer (e.g., cellulose-derived dialysis membranes), these materials can activate the oxidative metabolism of polymorphonuclear cells and macrophagesCitation1 in HD patients who require an increasing number of repeated extracorporeal circulations, then resulting in a series of cytotoxic effects commonly described as “oxidative stress”.Citation29,Citation30 This condition has been demonstrated to contribute to the pathogenesis of various complications such as cardiovascular diseases, beta-2 microglobulin-amyloidosis and protein malnutrition. To cope with this problem, dialyzers have been evolved into more biofriendly devices. In the 1990s, a cellulosic hollow-fiber dialyzers coated with vitamin E on the blood surface was developed by the Terumo Corporation with triple goals of better filtration, an improvement in biocompatibility and antioxidant bioactivity.Citation31 Soon afterward, vitamin E-coated polysulfone dialyzer was developed,Citation32 which has such excellent properties as good biocompatibility, clearance, permeability, and is easy to process. In addition, a number of clinical studies and meta-analysis have demonstrated that the anti-inflammatory and antioxidative effects of these vitamin E modified dialyzers reflected by the decreasing of circulating biomarkers (e.g., CRP, IL-6 and oxLDL levels).Citation11,Citation12
Oxidative and inflammatory events triggered by the extracorporeal treatments are believed to influence the entire uremic comorbidity. Beside cardiovascular disease, oxidative stress contributes importantly to uremic anemia.Citation21 In HD patients, due to impairment in defense mechanisms against oxidative stress,Citation33 the erythrocytes become a preferential target for HD-induced ROS overproduction, oxidative stress induces increased lipid peroxidation of the erythrocyte membrane then resulting in the resistance to erythropoietin and the shorter erythrocyte lifespan. Thus, using vitamin E modified membrane dialyzer was anticipated to decrease oxidation of the erythrocytes’ membrane lipids, improve abnormal erythrocyte morphology and prolong erythrocyte lifespan. In line with this, our pooled analysis showed that vitamin E coated dialyzer treatment did result in a decrease in EPO resistance index level (SMD, −0.24; 95% CI, −0.47 to −0.01; p = 0.04). However, in this meta-analysis, we were not able to find any significant effect of vitamin E-coated dialyzer therapy on EPO dose and HB levels in maintenance HD patients (p = 0.28; p = 0.74, respectively). It indicating that renal anemia associated with HD cannot be explained solely by the presence of inflammation and oxidative stress. In addition, some studies suggested that the improvements in EPO resistance after vitamin E-coated dialyzer therapy were not due to a reduction in systemic inflammation.Citation8,Citation16 On the other hand, previous studies have been suggested that supplementation of vitamin E also has a pro-oxidant action under special conditions such as other antioxidants (e.g. vitamin C) are deprived in HD patients,Citation34 it indicated that future larger and long-term randomized clinical trials are required to fully elucidate the clinical value of vitamin E-coated dialyzer plus appropriate replacement of ascorbic acid et al. lost during dialysis on renal anemia for HD patients.
Despite remarkable technological advances that dialysis treatment has achieved, there still exists many problems associated with prolonged HD such as protein malnutrition, atherosclerosis and accelerated cardiovascular disease.Citation35 It is reported that about 20–80% of the HD patients have a mildly to moderately malnourished status.Citation36,Citation37 In an inflammatory status like what we see in HD patients, inflammatory cytokines induced by bio-incompatible dialyzer membranes and dialysate bacterial contaminants can trigger an acute-phase reaction by the liver resulting in a decrease in serum albumin levels,Citation38 and the results of some cohort studies has also demonstrated the interaction of malnutrition and inflammation.Citation39,Citation40 As vitamin E-coated dialyzer treatment has been shown to decrease oxidative stress and inflammation status,Citation11,Citation12 it is proposed that vitamin E-coated dialyzer is likely to improve nutritional status by reducing inflammation status. And some clinical trials indicate vitamin E supplement can improve the malnutrition status in HD patients.Citation37 However, our pooled-analysis showed that vitamin E-coated dialyzer therapy did not result in a significant increase in serum albumin level (SMD, −0.64; 95% CI, −1.62 to 0.34; p = 0.20), it indicating that the hypoalbuminemia status not only induced by the presence of inflammation, but also related with the considerable losses of amino acids and/or protein during hemodialysis therapy.
About 40% of deaths of maintained hemodialysis patients have been attributable to atherosclerosis and consequent increased cardiovascular diseases,Citation41 although it still remains unclear whether HD per se affects the progression of the atherosclerosis, the patients undergoing maintained HD therapy tend to have more serious cardiovascular complications. It may be owing to severe oxidative stress induced by extracorporeal circulation and bioincompatible dialyzer membrane.Citation13 Many studies have confirmed that vitamin E-bonded hemodialyzer could significantly decrease oxidative stress status and improve the endothelial dysfunction,Citation13 therefore vitamin E-bonded dialyzer may be able to retrieve abnormality of atherosclerosis and lipid metabolism. But in this meta-analysis, we were not able to find significant effect of vitamin E-bonded dialyzer therapy on the IMT of the carotid artery (p = 0.09), which was a good indicator of atherosclerosis. In addition, the pooled analysis showed that vitamin E-coated dialyzer therapy had no effect on serum cholesterol and triglycerides (p = 0.71; p = 0.85 respectively). This result suggested that vitamin E-bonded dialyzer therapy might not be efficacious for controlling the HD-induced atherosclerosis and dyslipidemia, particularly when compared with other approved lipid-lowering drugs.Citation42
Several important potential study limitations to this systematic review should be acknowledged. Firstly, mostly included studies were of low quality and small-size, only two studies explained the randomization method, and most of studies were not blinded designed. In addition, some funnel plots for the key outcomes were asymmetric (), which suggested there was publication bias among these studies. Secondly, there were a few heterogeneities of baseline clinical features in these included RCTs or quasi-RCTs, such as different study duration and different types of conventional dialyzer, we should acknowledge that the accuracy of the pooled analytical results might be influenced. Thirdly, most included researches reported short-term (less than 1 year) outcomes of vitamin E-coated dialyzer treatment, and there were controversial in the subgroup-analysis whether vitamin E-coated dialyzer was beneficial for renal anemia and EPO resistance. So this result needed to be confirmed by more larger and long-term randomized clinical trials. Finally, although we observed some surrogate endpoints (HB and EPO dose et al.), while the studies did not examine hard clinical endpoints such as morbidity or mortality, thereby limiting the clinical relevance of our findings.
In summary, this meta-analysis indicates vitamin E-coated dialyzer may reduce the EPO resistance, but there is no conclusive evidence suggest a significant advantage in renal anemia, malnutrition, dyslipidemia and atherosclerosis status conferred by the use of vitamin E-coated dialyzer versus conventional dialyzers. As this report is merely an exploratory system review, future high-quality RCTs aimed at assessing the effects of vitamin E-coated dialyzer are warranted.
Declaration of interest
None of the authors is in any condition that may represent a potential conflict of interest.
This study was in part supported by the Natural Science Foundation of Hunan Province (NO. 13JJ3033), and the Scientific and Technological Project of Hunan Province (NO. 2011FJ3217).
References
- Bufano G, Usberti M, Mandolfo S, Malberti F, Piroddi M, Galli F. Von Willebrand factor and autoantibodies against oxidized LDL in hemodialysis patients treated with vitamin E-modified dialyzers. Int J Artif Organs. 2004;27(3):214–221
- Libetta C, Sepe V, Esposito P, Galli F, Dal Canton A. Oxidative stress and inflammation: Implications in uremia and hemodialysis. Clin Biochem. 2011;44(14–15):1189–1198
- Huang KC, Yang CC, Hsu SP, et al. Electrolyzed-reduced water reduced hemodialysis-induced erythrocyte impairment in end-stage renal disease patients. Kidney Int. 2006;70(2):391–398
- Lemoine S, Fournier T, Kocevar G, et al. Dialysis. Protein-energy wasting, inflammation and oxidative stress. Nephrol Dial Transplant. 2014;29(Suppl 3):iii287–iii303
- Elshamaa MF, Sabry S, Nabih M, Elghoroury EA, El-Saaid GS, Ismail AA. Oxidative stress markers and C-reactive protein in pediatric patients on hemodialysis. Ann Nutr Metab. 2009;55(4):309–316
- Panichi V, Scatena A, Migliori M, Marchetti V, Paoletti S, Beati S. Biomarkers of chronic inflammatory state in uremia and cardiovascular disease. Int J Inflam. 2012;2012:360147
- Danielski M, Ikizler TA, McMonagle E, et al. Linkage of hypoalbuminemia, inflammation, and oxidative stress in patients receiving maintenance hemodialysis therapy. Am J Kidney Dis. 2003;42(2):286–294
- Panichi V, Rosati A, Paoletti S, et al. A vitamin E-coated polysulfone membrane reduces serum levels of inflammatory markers and resistance to erythropoietin-stimulating agents in hemodialysis patients: Results of a randomized cross-over multicenter trial. Blood Purif. 2011;32(1):7–14
- Marques de Mattos A, Marino LV, Ovidio PP, Jordao AA, Almeida CC, Chiarello PG. Protein oxidative stress and dyslipidemia in dialysis patients. Ther Apher Dial. 2012;16(1):68–74
- Wratten ML, Galaris D, Tetta C, Sevanian A. Evolution of oxidative stress and inflammation during hemodialysis and their contribution to cardiovascular disease. Antioxid Redox Signal. 2002;4(6):935–944
- Sosa MA, Balk EM, Lau J, et al. A systematic review of the effect of the excebrane dialyser on biomarkers of lipid peroxidation. Nephrol Dial Transplant. 2006;21(10):2825–2833
- Yang SK, Xiao L, Xu B, Xu XX, Liu FY, Sun L. Effects of vitamin E-coated dialyzer on oxidative stress and inflammation status in hemodialysis patients: A systematic review and meta-analysis. Ren Fail. 2014;36(5):722–731
- Kobayashi S, Moriya H, Aso K, Ohtake T. Vitamin E-bonded hemodialyzer improves atherosclerosis associated with a rheological improvement of circulating red blood cells. Kidney Int. 2003;63(5):1881–1887
- Mandolfo S, Corradi B, Bucci R, Farina M, Pilolli F, Galli F. Evaluation of the impact of a new synthetic vitamin E-bonded membrane on anemia and rHuEPO requirement in ESRD patients with central venous catheters: A pilot study. Int Urol Nephrol. 2012;44(5):1493–1500
- Sanaka T, Mochizuki T, Kinugasa E, et al. Randomized controlled open-label trial of vitamin E-bonded polysulfone dialyzer and erythropoiesis-stimulating agent response. Clin J Am Soc Nephrol. 2013;8(6):969–978
- Lines SW, Carter AM, Dunn EJ, Lindley EJ, Tattersall JE, Wright MJ. A randomized controlled trial evaluating the erythropoiesis stimulating agent sparing potential of a vitamin E-bonded polysulfone dialysis membrane. Nephrol Dial Transplant. 2014;29(3):649–656
- Higgins JP. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; March 2011. Available at: http://www.cochrane-handbook.org
- Review Manager (RevMan) [Computer program]. The Nordic Cochrane Centre. Version 5.3. Copenhagen: The Cochrane Collaboration; June 2014. Available from: http://tech.cochrane.org/revman/
- Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634
- Baragetti I, Furiani S, Vettoretti S, et al. Role of vitamin E-coated membrane in reducing advanced glycation end products in hemodialysis patients: A pilot study. Blood Purif. 2006;24(4):369–376
- Andrulli S, Di Filippo S, Manzoni C, et al. Effect of synthetic vitamin E-bonded membrane on responsiveness to erythropoiesis-stimulating agents in hemodialysis patients: a pilot study. Nephron Clin Pract. 2010;115(1):c82–c89
- Clermont G, Lecour S, Cabanne JF, et al. Vitamin E-coated dialyzer reduces oxidative stress in hemodialysis patients. Free Radic Biol Med. 2001;31(2):233–241
- Kirmizis D, Papagianni A, Belechri AM, Memmos D. Effects of vitamin E-coated membrane dialyser on markers of oxidative stress and inflammation in patients on chronic hemodialysis. Nephrol Dial Transplant. 2011;26(7):2296–2301
- Morimoto H, Nakao K, Fukuoka K, et al. Long-term use of vitamin E-coated polysulfone membrane reduces oxidative stress markers in hemodialysis patients. Nephrol Dial Transplant. 2005;20(12):2775–2782
- Nakamura T, Kawagoe Y, Matsuda T, et al. Effects of LDL apheresis and vitamin E-modified membrane on carotid atherosclerosis in hemodialyzed patients with arteriosclerosis obliterans. Kidney Blood Press Res. 2003;26(3):185–191
- Usberti M, Gerardi G, Bufano G, et al. Effects of erythropoietin and vitamin E-modified membrane on plasma oxidative stress markers and anemia of hemodialyzed patients. Am J Kidney Dis. 2002;40(3):590–599
- Al-Jondeby MS, Cabaguing IT, Pajarillo AA, et al. Comparative crossover controlled study using polysulphone and vitamin E coated dialyzers. Saudi Med J. 2003;24(3):265–268
- Eiselt J, Racek J, Trefil L, Opatrny K Jr. Effects of a vitamin E-modified dialysis membrane and vitamin C infusion on oxidative stress in hemodialysis patients. Artif Organs. 2001;25(6):430–436
- Akiyama S, Inagaki M, Tsuji M, et al. Comparison of effect of vitamin E-coated dialyzer and oral vitamin E on hemodialysis-induced Cu/Zn-superoxide dismutase. Am J Nephrol. 2005;25(5):500–506
- Sasaki M, Hosoya N, Saruhashi M. Development of vitamin E-modified membrane. Contrib Nephrol. 1999;127:49–70
- Sasaki M. Development of vitamin E-modified polysulfone membrane dialyzers. J Artif Organs. 2006;9(1):50–60
- Cristol JP, Bosc JY, Badiou S, et al. Erythropoietin and oxidative stress in hemodialysis: Beneficial effects of vitamin E supplementation. Nephrol Dial Transplant. 1997;12(11):2312–2317
- Antoniadi G, Eleftheriadis T, Liakopoulos V, et al. Effect of one-year oral alpha-tocopherol administration on the antioxidant defense system in hemodialysis patients. Ther Apher Dial. 2008;12(3):237–242
- Rao P, Reddy GC, Kanagasabapathy AS. Malnutrition-inflammation-atherosclerosis syndrome in Chronic Kidney disease. Indian J Clin Biochem. 2008;23(3):209–217
- de Mutsert R, Krediet RT. Malnutrition, inflammation and atherosclerosis (MIA-syndrome) in dialysis patients. Ned Tijdschr Geneeskd. 2006;150(37):2023–2027
- Ahmadi A, Mazooji N, Roozbeh J, Mazloom Z, Hasanzade J. Effect of alpha-lipoic acid and vitamin E supplementation on oxidative stress, inflammation, and malnutrition in hemodialysis patients. Iran J Kidney Dis. 2013;7(6):461–467
- Kim Y, Molnar MZ, Rattanasompattikul M, et al. Relative contributions of inflammation and inadequate protein intake to hypoalbuminemia in patients on maintenance hemodialysis. Int Urol Nephrol. 2013;45(1):215–227
- Streja E, Kovesdy CP, Molnar MZ, et al. Role of nutritional status and inflammation in higher survival of African American and Hispanic hemodialysis patients. Am J Kidney Dis. 2011;57(6):883–893
- Rambod M, Bross R, Zitterkoph J, et al. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am J Kidney Dis. 2009;53(2):298–309
- Drueke TB, Massy ZA. Atherosclerosis in CKD: Differences from the general population. Nat Rev Nephrol. 2010;6(12):723–735
- Olyaei A, Greer E, Delos Santos R, Rueda J. The efficacy and safety of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors in chronic kidney disease, dialysis, and transplant patients. Clin J Am Soc Nephrol. 2011;6(3):664–678