Abstract
Aim: We aimed to investigate the prophylactic effects of trimetazidine (TMZ) against contrast-induced nephropathy (CIN) in rat kidneys. Methods and results: 28 Wistar rats were divided into 4 groups of 7 rats each (control (C), contrast media (CM) TMZ, trimetazidin + contrast media groups (TMZ + CM). The administration of TMZ solution was done on d2, d3 and d4. Fifth day, contrast media was administered at a single dose. On d6 scarification was performed. The oxidant/antioxidant parameters were measured and histopathological scores were performed in kidney tissues. Most of the histopathological scores were significantly higher in the CM group as compared to other groups. Moreover, the scores of the TMZ + CM and C groups were not statistically different. CM group, had significantly higher levels of MDA compared to the C and CM + TMZ groups (562.82 ± 38.15 vs. 419.15 ± 49.01 and 507.34 ± 14.16 01 nmol/mg protein respectively) (p < 0.001). CM group had significantly lower levels of SOD as compared to C, CM + TMZ and TMZ groups (p < 0.05). Conclusion: To the best of our knowledge, this study for the first time, histopathologically demonstrated the effectiveness of TMZ for the prevention of CIN.
Introduction
Diagnostic and therapeutic coronary interventions have been applied with a progressive procedural complexity as well as radiologic examinations and interventions. Together with the aging population, long procedural times and recurrent administration of iodinated contrast media for different purposes have led to an increase in the incidence of a complication known as contrast-induced nephropathy (CIN).
CIN is defined as the deterioration of renal function with an absolute increase in serum creatinine levels to ≥0.5 mg/d L or a ≥25% relative increase in serum creatinine levels within 48–72 h after exposure to contrast media. The final diagnosis of CIN should be judged after the exclusion of other reasons for renal impairment.Citation1 Usually serum creatinine levels begin to increase within 24–48 h after the contrast exposure, peak levels are seen at 3–5 d and they return to the baseline values at 7–10 d. However, CIN may require dialysis, prolonged hospital stay and has the potential to cause permanent renal damage. The incidence of CIN after the coronary angiography is about 15% and estimated in hospital mortality is about 25%.Citation2,Citation3
The exact pathogenesis of CIN is controversial but several mechanisms have been proposed. Renal vasoconstriction and renal hemodynamic disturbances, increased levels of endothelin, impaired nitric oxide production, endothelial dysfunction, direct cellular toxicity due to relatively high tissue osmolality, and reperfusion injury via free radical formation and oxidative stress are the suggested mechanisms.Citation4 Regarding these possible mechanisms, different pharmacological agents have been evaluated for the prevention of CIN in many trials. Many of them failed to show the benefit but hydration with isotonic saline is generally accepted to be useful in preventing CIN.Citation5,Citation6
A cellular anti-ischemic agent trimetazidine (TMZ) was previously demonstrated to have useful effects during ischemia-reperfusion at the cellular and the mitochondrial levels. In addition TMZ has strong antioxidant activity on various tissue preparations.Citation7,Citation8 On the basis of these anti-ischemic and antioxidant properties, Onbaşılı et al. previously demonstrated TMZ to be effective in preventing CIN.Citation9
Thus, we aimed to investigate the prophylactic effects of TMZ against CIN and its relation to oxidant/antioxidant status in rat kidney tissues in a contrast nephropathy induced rat model.
Materials and methods
Contrast medium
Low osmolar, non-ionic iohexol (Omnipaque 300, GE Healthcare, UK) consisting of iodine 300 mg mL−1 (Omnipaque 300 contains 647 mg of iohexol equivalent to 300 mg mL−1organic iodine) was used as CM.
Animals and experimental protocol
The study included 28 male Wistar rats aged 2–3-months that weighed 224–252 g. The rats were housed in a room maintained at 22–25 °C with a 12-h light/dark cycle (light cycle began at 0700). The rats were divided into four groups of seven rats each; control, CM, TMZ, and CM + TMZ groups. The rats received standard rat chow and had free access to tap water for 1 week. After 7 d of acclimation, the rats were housed in individual metabolism cages to collect 24-h urine and to record water intake (d 1). At the end of d 1, baseline blood samples were collected from the tail vein under ether anesthesia, and then analyzed for serum blood urea nitrogen (BUN) and creatinine. In addition, 24-h urine samples were collected. Blood samples were obtained and weight was measured between 0800 and 0900 to minimize circadian variation. Administration of TMZ solution was performed on d 2, d 3, and d 4. Aqueous solution of TMZ (10 mg kg−1 d−1) (Trimetazidine 20 mg, Servier) was administered via single gavage in the morning to the rats in the TMZ and CM + TMZ groups. The control group received 0.9% NaCl solution (IP) (equal to the volume of TMZ solution administered to the TMZ and CM + TMZ groups) instead of TMZ on d 2, d 3, and d 4. All groups were deprived of water for 72 h during this process and had unlimited access to standard rat chow.
On d 5 contrast media (Omnipaque 300 mg I mL−1, GE Healthcare) was administered under ether anesthesia as a single IV dose of 10 mL kg−1 into the tail vein over the course of 5 min in the CM and CM + TMZ groups.Citation10 On d 6 of the experiment, 24-h urine and serum samples were again collected.Citation11 Final blood samples were collected from the tail vein under ether anesthesia. After scarification, the right kidneys were removed and fixed in 10% formalin for histopathologic evaluation. The left kidneys were dissected to measure superoxide dismutase activity (SOD) and catalase (CAT) activity, and glutathione (GSH) and malondialdehyde (MDA) levels.
The study protocol was approved by the University of Adnan Menderes Animal Ethics Committee and all chemicals were purchased from Sigma Chemical (Germany). The study received partial support from the Adnan Menderes University Scientific Research Projects Commission (TPF-09004). The study followed the ethical rules of European Commission guidelines for the animals.
Sample collection, analysis and definition of CIN
On d1 and at the end of the experiment urine samples and blood samples were collected into tubes and analysis were done in the same day. The serum creatinine concentration was measured colorimetrically (Architect C-8000, Abbott, IL); CIN was defined as an increase in the serum creatinine concentration of 0.5 mg dL−1 or ≥25%, as compared to baseline.Citation12 Creatinine clearance was calculated according to Moller et al.Citation13
Tissue MDA, CAT, GSH, and SOD measurement
At the end of the experiment the rats were sacrificed via cervical dislocation; the dissected kidneys were immediately rinsed in ice-cold phosphate-buffered saline. Tissues were homogenized in 10% 150 mM phosphate buffer (pH 7.4) in an ice bath using a stirrer (IKA overhead stirrer; Staufen, Germany) at 2000 rpm for 1 min (1/10 w/v). The homogenate was centrifuged (Nüve-Bench Top Centrifuge, NF 800 R, Ankara, Turkey) at 6000 × g for 10 min at 4 °C. The supernatant was frozen at −80 °C (Glacier Ultralow Temperature Freezer, NuAire Inc., Plymouth, MA) in aliquots until used.
The total protein level in the supernatant was measured spectrophotometrically (Shimadzu UV-1601, Kyoto, Japan) using a commercially available kit (Archem Diagnostic Ind. Co., İstanbul, Turkey); the results are expressed as nmol mg−1 of protein. Tissue homogenate was used to estimate lipid peroxidation based on measurement of the formation of thiobarbituric acid reactive substances (TBARS), according to Yoshioka et al.Citation14 The concentration of MDA was calculated spectrophotometrically and is expressed as nmol mg−1 of tissue protein.
CAT activity was measured based on the rate of H2O2 decomposition at 240 nm.Citation15 CAT activity is expressed as k mg−1 of tissue protein. GSH was measured spectrophotometrically according to Tietze; and are expressed as mg g−1 of tissue protein.Citation16 SOD was estimated based on the generation of superoxide radicals produced by xanthine on xanthine oxidase. SOD activity was measured then spectrophotometrically and the results are shown as U mg−1 of tissue protein.Citation17
Renal histology
Renal tissue was weighed and homogenized under standard conditions. For histopathological analysis, portions of right kidney tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. Samples were cut to sections 4 µm thick and stained with H&E (hematoxylin and eosin), and then were examined microscopically. Between 2 and 6 longitudinally and transversally cut sections from each animal were used for microscopic evaluation. Acute renal injury was scored semi-quantitatively. In tubular degeneration (TD), the cytoplasm of the proximal tubule epithelial cells at the outer and at the level of the deep renal cortex, stained bodies of various sizes and vacuolization containing acidophilus were considered. Scoring of TD was performed like this: Absence of TD refers to score 0; Mild TD that is defined as small and a few focus TD in immediately beneath the capsule (0–10%) refers to score 1; Moderate TD that is defined as few focal focus TD along the tubular segment (10–25%) refers to score 2; Severe TD that is defined as diffuse and significant TD along the tubular segment (25–50%) refers to score 3; and very severe TD that is defined as diffuse, significant TD along the tubuler segment (above 50%) refers to score 4.
Histopathological evaluation of kidney glomeruli, tubules, interstitium, and arteries was performed by a pathologist using the following scale: 0 = normal (0–5% involvement); 1 = mild (6–25% involvement); 2 = moderate (26–75% involvement); 3 = severe (76–100% involvement).
Statistical analysis
Biochemical parameters, kidney weight, and histopathological findings were checked for normal distribution using the Shapiro–Wilk test and for homogeneity of variance using Levene’s test. Data were compared between groups using Kruskal–Wallis analysis of variance (ANOVA) or one-way ANOVA, according to whether or not data were normally distributed. Post hoc multiple comparisons were performed using the Mann–Whitney U test with Bonferroni correction or Duncan’s test. Serum creatinine, creatinine clearance, and body weight were compared using the paired-sample t test. All analyses were performed using SPSS 14 for Windows. The level of statistical significance was set a p < 0.05. All data are expressed as mean ± standard error (SE).Citation18
Results
Serum creatinine and creatinine clearance
The serum creatinine and creatinine clearance levels in each group are shown in . Creatinine clearance and the serum creatinine level in the TMZ, CM + TMZ, and control groups did not differ significantly between baseline and d5, whereas in the CM group creatinine clearance was significantly lower and the serum creatinine level was significantly higher (p < 0.05) ().
Table 1. Serum creatinine and creatinine clearance levels in each group.
Body and tissue weights
Body weight was significantly lower in all 3 groups on d 3, after the dehydration phase (p < 0.05). At the end of the experiment kidney weight did not significantly differ from the other groups (p < 0.05).
Tissue MDA, CAT, GSH, and SOD level
SOD and CAT activity, and GSH and MDA levels in the renal tissue of the rats with CIN are given in . SOD activity was significantly lower in the CM group than in the TMZ, CM + TMZ, and control groups (p < 0.05) (). There were not any significant differences in SOD activity between the TMZ, CM + TMZ, and control groups (p > 0.05) (). In the CM group the MDA level was significantly higher than in the control group (p < 0.001) (). Moreover, the MDA level was higher in the CM group than in the TMZ + CM group (562.82 ± 38.15 nmol mg−1 of protein vs. 507.34 ± 14.16 01 nmol mg−1 of protein), but the difference was not significant (p > 0.05) (). There were not any significant differences in the MDA level between the CM + TMZ and control groups (). The MDA level was significantly lower in the TMZ group than in the other three groups (p < 0.001).
Table 2. SOD and CAT activity, and GSH and MDA levels in all four groups.
CAT activity did not differ significantly between all 4 groups (); however, CAT activity in the CM + TMZ group was higher than that in the CM group (0.71 ± 0.22 mg−1 of protein vs. 0.54 ± 0.19 k mg−1 of protein) (). In the TMZ group the GSH level was significantly higher than in the other three groups (p < 0.05). There were not any significant differences in the GSH level between the CM, CM + TMZ, and control groups ().
Histopathological results
Renal section of the CM group is shown in and the histopathological scores of all groups are shown in . Glomerular sclerosis and interstitial fibrosis were not observed in any of the groups. Expansion of Bowman's capsule, tubule epithelium degeneration, tubule epithelium necrosis, and interstitial infiltration scores were significantly higher in the CM group than in the other three groups ().
Figure 1. (a) Mild congestion (arrow) in the renal tubules image of control group (H&E, ×200). (b) Expansion of Bowman’s capsule (arrowhead), tubular dilatation and degeneration (arrows), and inflammation (asterisks) in renal tissue image of the contrast medium group (H&E, ×200). (c) Mild congestion (arrow) in renal tissue image of the TMZ group (H&E, ×200). TMZ: Trimetazidine. (d) Congestion (long arrow) and mild dilatation in tubular epithelium (small arrows) in renal tissue image of the TMZ + CM group (H&E, ×400). TMZ + CM: Trimetazidine + Contrast medium.
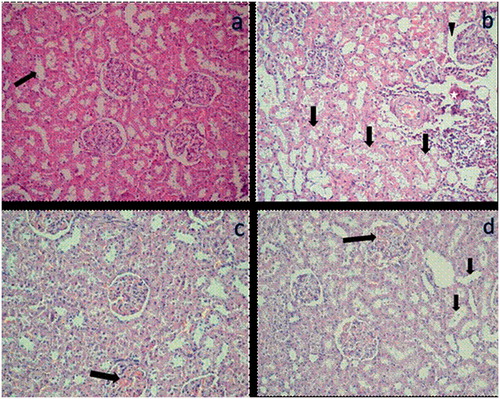
Figure 2. Histopathological findings in renal tissue. Expansion of Bowman’s capsule, tubule epithelium degeneration, tubule epithelium necrosis, tubule dilatation, interstitial infiltration and vascular congestion scores for each group. a and b: Each letter indicates statistically significant differences in the same row. **p < 0.01, ***p < 0.001.
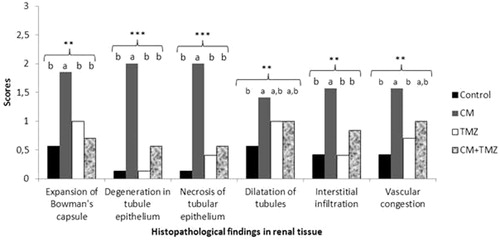
Moreover, tubule dilatation and vascular congestion scores in the CM group were significantly higher than those in the control group (); however, tubule dilatation and vascular congestion scores in the CM group did not differ significantly from those in the CM + TMZ group. In addition, all other histopathological scores in the CM group were significantly higher than those in the TMZ group, except for the tubule dilatation score (). Of note, none of the histopathological scores differed significantly between the CM + TMZ and control groups (). Histopathological scores in the TMZ and control groups did not differ significantly ().
Discussion
The most important finding of the present study is that the incidence of CIN was significantly lower in the CM + TMZ group; in addition, it was shown that the antioxidant properties of TMZ might have been responsible. Moreover, to the best of our knowledge the present study is the first to histopathologically demonstrate the effectiveness of TMZ in preventing CIN. Another finding of note in the present study was the lack of significant difference in any histopathological score between the CM + TMZ and control groups. In contrast, all histopathological scores in the CM group were significantly higher than those the control group. In addition, there were significant differences in serious pathological findings, including expansion of Bowman’s capsule, tubule epithelium degeneration, tubule epithelium necrosis, and interstitial infiltration between the CM and CM + TMZ groups.
The precise mechanism by which CIN occurs remains unclear, though it is thought to be multifactorial. The direct toxic effects of iodinated contrast agents in conjunction with changes in renal hemodynamics facilitate renal damage. Canine and rat models of CIN showed that use of CM produces oxygen free radicals that are cytotoxic.Citation19 Additionally, these free radicals are thought to cause apoptosis in renal tubule and glomerular cells.Citation9 Sandhu et al. reported earlier that the urinary MDA/creatinine ratio increased following CM infusion and that there might be a relationship between CM infusion and free radical generation.Citation20 The present study’s findings are in agreement with these data, as the MDA level significantly increased and SOD activity significantly decreased after administration of CM in the CM group, as compared to the control group. These findings suggest that oxygen radicals play an important role in this particular nephrotoxicity model. The observed increase in the MDA level indicates that use of CM resulted in oxidative stress in rat kidney tissues.
The literature includes many studies on various agents that were tested in vitro and in vivo for the prevention of CIN and the experimental models for the aggravation of CIN includes water deprivation, as we used in our present study, or induction hypercholesterolemic diet before exposure to CM.Citation21–23 However, the findings of most of the studies are inconsistent, except for the positive effect of hydration or hydration in combination with N-acetylcysteine (NAC) or sodium bicarbonate before the procedure.Citation24 Recently, Onbaşılı et al. were the first to report that TMZ—a cellular anti-ischemic agent—was effective in preventing CIN in patients undergoing coronary procedures.Citation9 Moreover, Rahman et al. confirmed those findings in a study that included 400 patients undergoing coronary angiography;Citation25 however, neither study was able to identify the physiopathological mechanism of action or was designed to include histopathological findings. As such, the present study aimed to histopathologically analyze the effect of TMZ in preventing CIN and to identify the probable biochemical mechanism of action. Reports of the strong association between CIN and oxygen free radicals led us to focus on the antioxidant properties of TMZ. TMZ is a piperazine derivative that inhibits 3-ketoacyl-CoA-thiolase. It reduces fatty acid oxidation and stimulates glucose use. During hypoxia and ischemia TMZ maintains cellular functions by decreasing cellular acidosis and increasing ATP production.Citation26,Citation27 TMZ was shown to have antioxidant properties.Citation28,Citation29 Under ischemic conditions TMZ reduces the loss of intracellular K+ induced by oxygen free radicals and protects cellular membrane.Citation30 It was shown that the plasma MDA level decreased in response to pre-treatment with TMZ before coronary artery bypass surgery.Citation31 Another study reported that long-term administration of TMZ significantly reduced superoxide anion generation and the MDA level following ischemia-reperfusion.Citation32 The potent antioxidant effect of TMZ has been shown in renal, myocardial, and hepatic ischemia-reperfusion injury models.Citation33,Citation34
The CM group in the present study had significantly lower SOD activity than the CM + TMZ group. The CM group also had a higher MDA level than the CM + TMZ group, but the difference was not significant. As compared to the CM group, CAT activity and the GSH level were also higher (not significantly) in the CM + TMZ group. Another noteworthy finding of the present study is the lack of significant difference in SOD and CAT activity, and MDA and GSH levels between the CM + TMZ and control groups. These findings together with the concordant histopathological findings suggest that TMZ might play an important role in preventing CIN, perhaps due to its antioxidant properties. Nonetheless, some properties of TMZ other than its antioxidant effect might have contributed to its effectiveness in preventing CIN in the present study.
Conclusion
CIN is a serious complication associated with high rates of morbidity and mortality. Administration of TMZ may help prevent CIN and may reduce the need for hemodialysis and extended hospitalization, and the incidence of permanently impaired renal function.
Declaration of interest
The authors declare no conflicts of interests. The authors alone are responsible for content and writing of this article.
Notes
*The abstract of this report was admitted as a poster presentation in ESC 2013.
References
- Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105(19):2259–2264
- Murphy SW, Barrett BJ, Parfrey PS. Contrast nephropathy. J Am Soc Nephrol. 2000;11:177–182
- Gruberg L, Mehran R, Dangas G, et al. Acute renal failure requiring dialysis after percutaneous coronary interventions. Cathet Cardiovasc Intervent. 2001;52:409–416
- Tumlin J, Stacul F, Adam A, et al.; CIN Consensus Working Panel. Pathophysiology of contrast-induced nephropathy. Am J Cardiol. 2006;98:14K–20K
- Gami AS, Garovic VD. Contrast nephropathy after coronary angiography. Mayo Clin Proc. 2004;79:211–219
- Aydoğdu S. Contrast-induced nephropathy. Turk Kardiyol Dern Ars. 2013;41(1):28–30
- Elimadi A, Settaf A, Morin D, et al. Trimetazidine counteracts the hepatic injury associated with ischemia-reperfusion by preserving mitochondrial function. J Pharmacol Exp Ther. 1998;286:23–28
- Aubert A, Bernard C, Clauser P, Harpey C, Vaudry H. Effect of pnenazine methosulfate on electrophysiological activity of the semisircular canal: antioxidant properties of trimetazidine. Eur J Pharmacol. 1989;174:215–225
- Onbasili AO, Yeniceriglu Y, Agaoglu P, et al. Trimetazidine in the prevention of contrast-induced nephropathy after coronary procedures. Heart. 2007;93(6):698–702
- Singh AP, Junemann A, Muthuraman A, et al. Animal models of acute renal failure. Pharmacol Rep. 2012;64(1):31–44
- Colbay M, Yuksel S, Uslan I, et al. Novel approach for the prevention of contrast nephropathy. Exp Toxicol Pathol. 2010;62:81–89
- Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJç. Nephrotoxic effects in high-risk patients undergoing angiography. N Eng J Med. 2003;348(6):491–499
- Moller E, McIntosh JR, VanSlyke DD. Studies of ureaexcretion. II. Relationshipbetweenurinevolumeandthe rate of ureaexcretionby normal adults. J Clin Invest. 1929;6:427–465
- Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol. 1979;135:372–376
- Bergmeyer H, Gawehn K, Grasse M. Enzyme as biochemical reagents. In: Bergmeyer HV, ed. Methods of Enzyme Analysis. New York: Academic Press; 1974:438–458
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Ann Biochem. 1969;27:502–522
- Sun Y, Oberley, LW, Li Y. A simple for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500
- Conover WJ, ed. Some methods based on ranks, section 5.2 several independent samples. In: Practical Nonparametric Statistics 2nd ed., Chapter 5. New York, NY: John Wiley & Sons; 1980: 229–239
- Hizoh I, Haller C. Radiocontrast-induced renal tubular cell apoptosis: hypertonic versus oxidative stress. Invest Radiol. 2002;37:428–434
- Sandhu C, Newman DJ, Morgan R, Belli AM, Oliveira D. The role of oxygen free radicals in contrast induced nephrotoxicity. Acad Radiol. 2002;9(Suppl 2):S436–S437
- Yang D, Lin S, Yang D, Wei L, Shang W. Effects of short- and long-term hypercholesterolemia on contrast-induced acute kidney injury. Am J Nephrol. 2012;35(1):80–89
- Andrade L, Campos SB, Seguro AC. Hypercholesterolemia aggravates radiocontrast nephrotoxicity: protective role of L-arginine. Kidney Int. 1998;53(6):1736–1742
- Boyacioglu M, Turgut H, Akgullu C, Eryilmaz U, Kum C, Onbasili OA. The efficient of L-carnitine on oxidative stress responses of experimental contrast-induced nephropathy in rat. J Vet Med Sci. 2013 Aug 20. [Epub ahead of print]
- Briguori C, Airoldi F, D’Andrea D, et al. Renal insufficiency following contrast media administration trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–1217
- Rahman MM, Haque SS, Rokeya B, Siddique MA, Banerjee SK, Ahsan SA. Trimetazidine in the prevention of contrast induced nephropathy after coronary angiogram. Mymensingh Med J. 2012;21(2):292–299
- Harpey C, Clauser P, Labrid C, Freyria JL, Poirier JP. Trimetazidine, a cellular anti-ischemic agent. Cardiovasc Drug Rev. 1989;6:292–312
- Stanley WC, Lopaschuck GD, Hall JL, Mccormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischemic conditions: potential for pharmacological interventions. Cardiovasc Res. 1997;33:243–257
- Maupoil V, Rochette L, Tabard A, Clauser P, Harpey C. Evolution of free radical formation during low-flow ischemia and reperfusion in isolated rat heart. Cardiovasc Drugs Ther. 1990;4:791–795
- Guarnieri C, Muscari C. Beneficial effects of trimetazidine on mitochondrial function and superoxide production in the cardiac muscle of monocrotaline-treated rats. Biochem Pharmacol. 1988;37:4685–4688
- Guarnieri C, Muscari C. Effect of trimetazidine on mitochondrial function and oxidative damage during reperfusion of ischemic hypertrophied myocardium. Pharmacology. 1993;46:324–331
- Fabiani JN, Ponzio O, Emerit I, et al. Cardioprotective effect of trimetazidine during coronary artery graft surgery. J Cardiovasc Surg. 1992;33:486–491
- Ruiz-Meana M. Trimetazidine, oxidative stress, and cell injury during myocardial reperfusion. Rev Esp Cardiol. 2005;58:895–897
- Ozden A, Aybek Z, Saydam N, et al. Cytoprotective effect of trimetazidine on 75 min warm renal ischemia-reperfusion injury in rats. Eur Surg Res. 1998;30:227–234
- Tsimoyiannis EC, Moutesidou KJ, Moschos CM, Karayianni M, Karkabounas S, Kotoulas OB. Trimetazidine for prevention of hepatic injury induced by ischemia and reperfusion in rats. Eur J Surg. 1993;159:89–93

