Abstract
Although enzymuria tends to be associated to renal injury, there are no studies that have evaluated the presence of the enzyme gamma-glutamyl transpeptidase (GGT) spectrophotometry in the urine using a non-nephrotoxic agent (Nerium oleander) in order to evaluate the possibility of false positive results. The urinary GGT/urinary creatinine concentration ratio (uGGT/uCr) of 10 healthy dogs was calculated and posteriorly confronted with data from clinical evaluation, hematological and serum biochemical profiles, creatinine clearance (CrC), urinalysis, urine protein/creatinine ratio (UPC), electrocardiogram, systemic blood pressure (SBP) and light and electron microscopy. The results for kidney histology, SBP, UPC and CrC were not significantly different in any of the time-points analyzed. However, uGGT/uCr was significantly higher when measured 4 hours and 24 hours after administration of N. oleander. The measurement of the urinary GGT enzyme, as performed in many studies, yielded false positive results in dogs poisoned by a non-nephrotoxic agent.
Introduction
Increased activities of some urinary enzymes suggest injury to tubular cells or indicate increased lysosomal activity without cell disruption. However, the majority of enzymes present in urine are brush border enzymes such as gamma-glutamyl transpeptidase (GGT). Increased excretion of these proteins implies injury to the brush border membrane with loss of microvillus structure. Loss of a significant fraction of the microvillus surface area also leads to reduced reabsorption and increased excretion of filtered proteins.Citation1
GGT is a glycoprotein which is attached to the external surface of various cell types. In the kidney, the primary site for GGT activity is the outer surface of microvillus membranes of the proximal tubule cells.Citation1 In the dog, GGT is relatively stable at room temperature and at 4 °C and may be measured in urine supernatant without prior removal of enzyme inhibitors.Citation2
Since many enzymes are released from the kidney tissue into the urine when there is kidney injury, urinary enzymes have recently been added to the list of markers of nephrotoxicity. Many reports have been published on the changes in urinary enzyme activity induced by drug nephrotoxicity,Citation3 toxic kidney injury,Citation2 or ischemic kidney damage.Citation4
Enzymuria tends to be associated to renal injury and this leads to the hypothesis that monitoring tubular enzymuria could predict or detect early loss of renal function.Citation1 However, there are no studies for evaluation of urinary GGT by spectrophotometry using a non-nephrotoxic agent in order to investigate the presence of false positivity.
Oleanders are evergreen flowering shrubs that belong to the Dogbane family, Apocynaceae. Nerium oleander (N. oleander) contains cardenolide within its tissues, which are capable of exerting positive inotropic cardiac effects in animals and humans. The basis for the physiological action is similar to those of the classic digitalis glycosides, mainly causing inhibition of the cell membrane Na+,K+ATPase activity. Toxic exposure of humans and wildlife animals to oleander occurs regularly in areas where these plants grow. The cardiac glycosides present in N. oleander include oleandrin, folinerin, and digitoxigenin.Citation5
Humans, dogs, cats, goats and monkeys have been found to be very sensitive to the cardioactive glycosides present in oleander leaves.Citation5 Nevertheless, the effects can be reversed with digoxin-specific Fab antibody fragments.Citation6 These data show that N. oleander is a toxic plant that exhibits predominantly cardiotoxic effects. The objective of this study was to evaluate the presence of false positivity of GGT enzyme in the urine of dogs confirmed through renal function tests and kidney histology after poisoning with a non-nephrotoxic substance.
Materials and methods
Ten healthy dogs (5 males and 5 females) with ages ranging from 3 to 6 years and weight ranging from 10.0 to 25.0 kg were used in this study. The study was approved by the Ethics Committee of the Universidade Estadual Paulista (UNESP), Jaboticabal, Brazil (006357-09).
Animals were kept under the same conditions for 14 days, with water ad libitum and food provided twice a day and were divided into two groups. Percutaneous renal biopsy using ultrasound guidance was performed before administration of the plant (baseline) using a Tru-cut® device (Trucut US Biopsy – Franklin, MA) with 18 G biopsy needles, and samples were processed for light microscopy. Twenty-four hours (Group 1, n = 5) and 48 hours (Group 2, n = 5) after N. oleander was administered to the animals, renal biopsies were repeated. All biopsies contained six glomeruli or more and were performed in order to investigate the possibility of renal lesions at the moment of peak concentration of urinary GGT.
Renal tissue for light microscopy was fixed by immersion in Bouin’s solution, dehydrated in alcohol and embedded in paraffin. Four-micrometer-thick sections were stained with hematoxylin and eosin (HE), periodic acid-Schiff (PAS), periodic acid-silver methenamine, and Masson's trichrome stains. The histomorphological features of the renal parenchyma were evaluated including glomeruli, tubules, interstitium and vessels. A comparison of the condition of renal tubules between dogs at two different biopsy time points was performed by evaluating the thickness of tubular basement membrane and uniformity of the tubular circumference.
Four randomly biopsies samples were chosen to electron microscopy. They were fixed by immersion in 2.5% glutaraldehyde in 0.2 M sodium cacodylate (pH 7.3) for 2 hours. And postfixed in em tetróxido de ósmio a 1%, buffered with 0.2 M for 2 hours. After it was dehydrated in a graded series of acetone, and embedded in Araldite 502 (Polysciences Inc., Warrington, PA, EUA).
The choice for amount and concentration of N. oleander leaves was based on a pre-experimental study which demonstrated that a dose of 0.25 g/kg administered crushed with the feed was able to cause cardiac alterations (electrocardiographic) without causing important clinical alterations.
For all dogs admitted to the study, blood samples were collected once a day and urine four times a day for up to 14 days (10 previously and 4 after administration of the plant). Creatinine clearance measurements were performed with 24-hour urine collection.
Urine specimens were processed immediately after collection and centrifugation. The sediment was examined and supernatant aliquots were taken for determination of creatinine (by the Jaffé reaction), protein, and urinary GGT activity by spectrophotometry. GGT indices were determined with the urinary GGT enzyme activity to urinary creatinine ratio (uGGT/uCr).
Clinical condition, hematological and serum biochemical profiles, creatinine clearance, urinalysis, urine protein/creatinine ratio (UPC), electrocardiogram, systemic blood pressure and urinary GGT/urinary creatinine concentration ratio were monitored in all animals.
The study followed a randomized design. Animal exclusion criteria included presence of urinary tract infection or other comorbid conditions. Results were subjected to analysis of variance (ANOVA) with time as the variation factor. To compare mean values, the multiple comparison Tukey–Kramer test was used. Differences were considered significant at p < 0.05. Calculations were performed using SAS® software (Statistical Analysis System, Jaboticabal, SP, Brazil).
Results
Animals exhibited vomiting (n = 10), sialorrhea (n = 8), nausea, apathy, conjunctival congestion (n = 7), mild dehydration (n = 5), abdominal pain, tremors (n = 3), and tenesmus (n = 1) 27 to 75 minutes after administration of N. oleander with the feed. Twelve hours after, nine dogs had consumed all the feed offered and one dog consumed less than the usual expected.
There was significant decrease in heart rate in all animals 4 hours after administration. All dogs exhibited arrhythmias in the first 24 hours, which were classified as sinus bradycardia (n = 4), second degree atrioventricular block (n = 2), paroxysmal ventricular tachycardia (n = 2), premature ventricular complex (n = 2), and sinus tachycardia (n = 1). After 48 hours no electrocardiographic abnormalities was identified.
Serum biochemical parameters (creatinine, ALT, ALP, GGT, total protein, albumin, sodium, potassium, and ionized calcium) did not exhibit significant difference before and after poisoning. The standard laboratory measurements of renal function and tubular markers are summarized in .
Table 1. Mean values ± standard deviation for systemic blood pressure (SBP), UPC, creatinine clearance (CrC), and median values ± interquartile range for urinary GGT enzyme activity to urinary creatinine ratio (uGGT/uCr) of dogs from G1 and G2 at baseline (T0), 4 hours (T4), 24 hours (T24), and 48 hours (T48) after experimental administration of N. oleander.
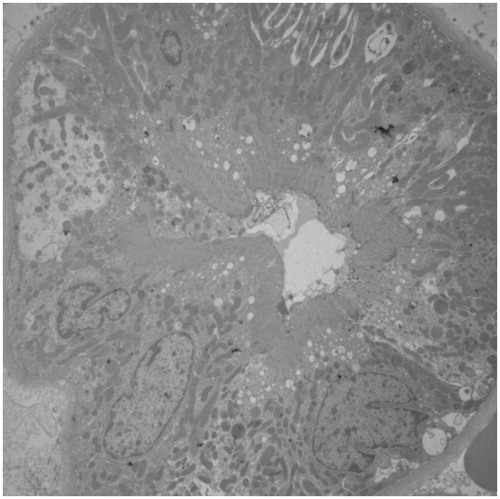
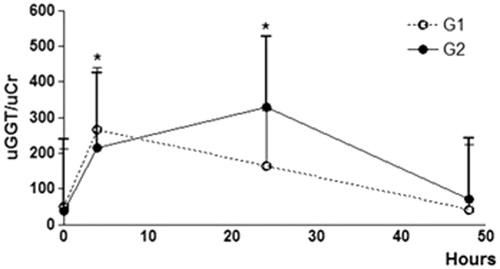
No difference was found in renal histology, systolic blood pressure, UPC, and CrC at any of the time points analyzed, while uGGT/uCr was significantly higher 4 hours and 24 hours after administration of the plant (). No lesions were detected at the ultrastructural level ().
Figure 1. Graphical representation of urinary GGT enzyme activity to urinary creatinine ratio (uGGT/uCr) in healthy dogs (G1 = 5) and (G2 = 5) before and after administration of N. oleander. The uGGT/uCr ratio exhibited a significantly higher concentration at 4 and 24 hours compared to baseline (0 hours) and 48 hours (*p < 0.05).
Discussion
Most studies show that urinary biomarkers increase according to the degree of structural renal injury.Citation7 Furthermore, there are studies that imply increase in urinary GGT as a result of acute kidney injury, even though there is no histological proof of lesion.Citation1 Considering the scarcity of studies using non-nephrotoxic agents, we compared histological findings and renal function tests in dogs which received administration of N. oleander, in order to evaluate the exact relationship between structural and ultrastructural renal injury and urinary biomarkers.
Acute renal injuries usually lead to structural kidney damage and protein and casts show up in the urine after 2 days.Citation7 During the 4 days following administration of N. oleander, no alteration in urine, UPC, or creatinine clearance were seen, which show that the substance did not cause significant alterations in laboratory tests that evaluate renal function.
While previous studies have demonstrated that enzymuria can detect tubular injury,Citation2–4,Citation7 the present study demonstrates for the first time that the increase in urinary GGT does not necessarily correlate with alterations in kidney histology or in renal function tests. Therefore, the GGT dosage found by simple spectrophotometry, which has been used in several studies,Citation1,Citation2,Citation4,Citation8–10 can exhibit false positivity in dogs poisoned by N. oleander. This finding should be considered in order to avoid interpretation errors and unnecessary biopsy procedures.
Although the main route of cardiac glycosides excretion is through biliary excretion into the feces, the urine route is only a smaller route. The oleander plant contains about 30 different cardiac glycosides and others derived toxins.Citation5,Citation6 Maybe one of these substances can be measured in the urine by spectrophotometry as GGT.
Although a recent study in humans showed that urinary GGT concentration does not increase in dehydrated patientsCitation7, care was taken when choosing a dose of N. oleander that would cause cardiac alterations with few clinical effects to avoid renal alterations due to dehydration.
It is important to highlight that the animals were evaluated with laboratorial tests 10 days after the first biopsy (before administration of N. oleander) for posterior comparisons. During this period there was no increase in urinary GGT or any of the other parameters, which excludes the possibility of the biopsy procedure causing increase in urinary GGT after poisoning.
N. oleander was chosen as the toxic agent due to the fact that it is a cosmopolitan plant that can be easily found in homes, is the cause of many natural poisoning cases in various species,Citation11 and causes only cardiac alterations.Citation5,Citation6
It would be of great value to determine urinary GGT activity, as well as other urinary enzymes in future studies using non-nephrotoxic substances in order to investigate if urinary markers quantification can be altered by other substances present in the urine.
Declaration of interest
The authors have declared that no conflict of interest exists.
References
- Westhuyzen J, Endre ZH, Reece G, Reith DM, Saltissi D, Morgan TJ. Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol Dial Transplant. 2003;18:543–551
- Clemo FAS. Urinary enzyme evaluation of nephrotoxicity in the dog. Toxicol Pathol. 1998;26(1):29--32
- Harauchi T, Yoshizaki T. Method for determining urinary enzyme activities as nephrotoxic indicators in rats. Jpn J Pharmacol. 1990;54:205–215
- Cutrín JC, Zingaro B, Camandola S, Boveris A, Pompella A, Poli G. Contribution of γ glutamyl transpeptidase to oxidative damage of ischemic rat kidney. Kidney Int. 2000;57:526–533
- Langford SD, Boor PJ. Oleander toxicity: an examination of human and animal toxic exposures. Toxicology. 1996;109(1):1–13
- Camphausen C, Haas NA, Mattke AC. Successful treatment of oleander intoxication (cardiac glycosides) with digoxin-specific Fab antibody fragments in a 7-year-old child. Z Kardiol. 2005;94:817--823
- Nejat M, Pickering JW, Devarajan P, et al. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int. 2012;81:1254--1262
- Matteucci E, Gregori G, Pellegrini L, Navalesi R, Glampietro O. Effects of storage time and temperature on urinary enzymes. Clin Chem. 1991;37(8):1436–1441
- Palacio J, Liste F, Gascón M. Enzymuria as an index of renal damage in canine leishmaniasis. Vet Rec. 1997;140:477–480
- Uechi M, Uechi H, Nakayama T, et al. The circadian variation of urinary N-acetyl-β-D-glucosaminidase and γ-glutamyl transpeptidase in clinically healthy cats. J Vet Med Sci. 1998;60(9):1033–1034
- Aslani MR, Movassaghi AR, Janati-Pirouz H, Karazma M. Experimental oleander (Nerium oleander) poisoning in goats: a clinical and pathological study. Iran J Vet Res. 2007;8(1):58–63