Abstract
Oxidative stress and suppressed H2S production lead to increased renal vascular resistance, disturbed glomerular hemodynamics, and abnormal renal sodium and water handling, contribute to the pathogenesis and maintenance of essential hypertension in man and the spontaneously hypertensive rat. This study investigated the impact of H2S and tempol alone and in combination on blood pressure and renal hemodynamics and excretory functions in the SHR. Groups of WKY rats or SHR (n = 6) were treated for 4 weeks either as controls or received NaHS (SHR + NaHS), tempol (SHR + Tempol), or NaHS plus tempol (SHR + NaHS + Tempol). Metabolic studies were performed on days 0, 14, and 28, thereafter animals were anaesthetized to measure renal hemodynamics and plasma oxidative and antioxidant markers. SHR control rats had higher mean arterial blood pressure (140.0 ± 2 vs. 100.0 ± 3 mmHg), lower plasma and urinary H2S, creatinine clearance, urine flow rate and urinary sodium excretion, and oxidative stress compared to WKY (all p < 0.05). Treatment either with NaHS or with tempol alone decreased blood pressure and oxidative stress and improved renal hemodynamic and excretory function compared to untreated SHR. Combined NaHS and tempol therapy in SHRs caused larger decreases in blood pressure (∼20–22% vs. ∼11–15% and ∼10–14%), increases in creatinine clearance, urinary sodium excretion and fractional sodium excretion and up-regulated the antioxidant status compared to each agent alone (all p < 0.05). These findings demonstrated that H2S and tempol together resulted in greater reductions in blood pressure and normalization of kidney function compared with either compound alone.
Introduction
Spontaneously hypertensive rats (SHR) have been widely used as a model for human essential hypertension because of the similarity of the main cardiovascular characteristics. A large number of studies have provided evidence that altered renal function plays a key role in the pathogenesis of essential hypertension.Citation1,Citation2 Renal defects range from distorted glomerular hemodynamics to abnormal regulation of renal tubular sodium and water handling.Citation3 The renal insufficiency is due, in part, to the enhanced reactive oxygen species (ROS) particularly superoxide anion () in the blood vessels and kidneys of SHRsCitation4 which precedes the development of hypertension in SHRs.Citation5 There is emerging evidence that
acts as a direct renal vasoconstrictor and is antinatriuretic by directly acting on renal sodium transport.Citation6 Moreover,
can stimulate pressor systems, such as the renin–angiotensinCitation7 and sympathetic nervous system (SNS)Citation8 resulting in augmented renal sodium and water reabsorption. Experimental studies and observational findings suggest that therapies targeted to decreasing
production may improve the renal dysfunction associated with essential hypertension.Citation9,Citation10 Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) is a cell membrane-permeable ampholyte that dismutates
catalytically and facilitates hydrogen peroxide metabolism by a catalase-like action to limit formation of toxic hydroxyl radicals produced by the Fenton reaction.Citation11 The acute antihypertensive response to tempol is related to its in vitro superoxide dismutase (SOD)-mimetic activity.Citation12 However, the long-term outcome of tempol administration in relation to the kidney may well be a correction of salt sensitivity,Citation13 renal hypoxia,Citation4 and renal vasoconstriction.Citation14
Hydrogen sulfide (H2S) has attracted a great deal of interest as it can be generated endogenously by the action of two enzymes cystathionine beta synthase (CBS) and cystathionine gamma lyase (CSE) from l-cysteine.Citation15 H2S is a vasodilator moleculeCitation16 acting on vascular smooth muscle cells by opening KATP channels.Citation17 A significant down regulation of the H2S/CSE system has been reported in several hypertensive models.Citation18,Citation19 Furthermore, H2S supplementation protects the kidney from ischemia/reperfusion injury and improves renal function.Citation20 It has been reported that acute infusion of sodium hydrosulfide (NaHS), an exogenous hydrogen sulfide donor, into the renal artery increases urinary sodium excretion through both vascular and tubular actions in the kidney.Citation21 However, whether long-term exogenous H2S administration ameliorates the renal sodium retention associated with hypertension is not clear.
Previous studies have led to the view that impaired and defective vasodilatation in the renal vasculature due to increased oxidative stress as a primary contributor to the development and maintenance of hypertension in SHRs. We hypothesized that tempol being an antioxidant while hydrogen sulfide has been shown to be both vasodilator and natriuretic can interact in an additive or potentiating manner to improve blood pressure control, renal hemodynamics, and excretory functions of SHRs when given in combination. The approach taken was to measure the blood pressure, oxidative and antioxidant status, renal hemodynamics and renal excretory functions in WKY and SHRs over a 4-week period during the exogenous administration of hydrogen sulphide, tempol, or a combination of H2S and tempol.
Materials and methods
Experimental animals and protocol
Male (220–250 g body weight) WKY and SHR were obtained from the Animal Research Unit and Service Centre (ARASC) of Universiti Sains Malaysia. All the animals were housed in the same environmental conditions with free access to food (Gold Coin, Sdn. Bhd., Penang, Malaysia) and drinking water ad libitum. All the experimental procedures were carried out according to the guidelines of Universiti Sains Malaysia Animal Ethics Committee.
After 3 d of acclimatization, all rats were subjected to non-invasive blood pressure measurements (tail cuff method) using the CODA equipment (Kent Scientific Corporation, Torrington, CT) for a further three consecutive days to establish the basal blood pressures before starting the protocol. Only SHRs exhibiting systolic blood pressure of 150 mmHg or above were used. SHRs were randomly divided into four groups (n = 6 in each group) namely SHR, SHR + NaHS, SHR + Tempol, and SHR + NaHS + Tempol. Equal numbers of matching body weight and age WKY rats served as normotensive controls. Previous reports have demonstrated that chronic administration of tempol or sodium hydrosulfide (NaHS), a donor of exogenous hydrogen sulfide, had no effect in normotensive ratsCitation18,Citation22 and thus these experiments were not repeated in present study. Tempol (Sigma Aldrich, Selangor, Malaysia) was given at 30 mg/kg/d for 4 weeks and was administered in drinking water. This dose was reported to decrease the plasma or urinary 8-isoprostane level in hypertensive rats.Citation23,Citation24 NaHS (Sigma Aldrich, Selangor, Malaysia) was freshly prepared in saline and 56 μmol/kg was injected intraperitoneally at the same time daily for four 4 weeks.Citation18 Twenty-four hour urine collections were performed in metabolic cages before the start of treatment protocol, to establish the basal excretory variables, and then on days 14 and 28 of the experiment. Water intake, food intake, and body weight were also measured on each day of urine collection. Glomerular filtration rate (GFR) was calculated using creatinine clearance. Blood samples were collected on days 0, 14, and 28 and plasma was obtained following centrifugation at 3500 rpm for 10 min. Plasma and urine sample were stored at −30 °C for further biochemical analysis.
Invasive blood pressure and renal hemodynamics (acute experiment)
At the end of 28 d, the animals were subjected to the acute study. All the rats were fasted overnight and anesthetized with sodium pentobarbitone at 60 mg/kg (Nembutal®, Ceva, Santé Animale, Libourne, France) which was supplemented intravenously (15 mg/kg) when necessary. Tracheotomy was then performed to maintain a clear airway using an endotracheal cannula (PE 240, Portex, Kent, UK). The left jugular vein was catheterized (PE 50 tubing Portex Ltd., England, UK) to permit the infusion of supplementary anesthesia. The right carotid artery was cannulated (PE 50 tubing Portex Ltd.) and connected to a pressure transducer (P23 ID Gould, Statham Instruments, London, UK) linked to a data acquisition system (PowerLab®, ADInstruments, Colorado Springs, CO) through a Quad Amp (ADInstruments) using chart Pro (V.5.5) software (ADInstruments, Colorado Springs, CO). The kidney was exposed via a midline incision. A laser Doppler flow probe (OxyFlow, ADInstruments) was positioned on the dorsal surface of the posterior end of the exposed left kidney in order to measure renal cortical blood perfusion. The probe was connected to a laser Doppler flowmeter (ADInstruments) which was directly linked to the data acquisition system (PowerLab®, ADInstruments). The animals were allowed to stabilize for 1 h upon completion of the above surgical procedure. After the stabilization period, renal cortical blood perfusion, mean arterial pressure, systolic blood pressure, and heart rate were recorded continuously for 30 min. and averaged.
Biochemical analysis for oxidative stress markers
At the end of the acute protocol, 5 mL of arterial blood was obtained via the carotid artery cannula, centrifuged at 3500 rpm for 10 min to obtain plasma which was stored at −30 °C till analysis of antioxidant markers. Plasma total superoxide dismutase (T-SOD), malondialdehyde (MDA), total antioxidant capacity (T-AOC), and nitric oxide (NO) were measured using the spectrophotometric detection kit (Institute of Biological Engineering of Nanjing Jianchen, Nanjing, China). All the measurements were made according to the kit manufacturer’s instructions.
Biochemical analysis of stored plasma and urine samples
Plasma and urinary creatinine concentrations were measured spectrophotometrically (Jaffe’s reaction) while sodium and potassium concentrations were measured using a flame photometer (Jenway Ltd., Felsted, UK). H2S concentrations in the plasma or urine were measured spectrophotometrically and calculated against the calibration curve of standard H2S solutions (NaHS: 3.125–100 μM).Citation25 Creatinine clearance (Cr.Cl.), fractional excretion of sodium (FENa), and absolute urinary sodium excretion (UNa V) were calculated using the standard equations. Urinary sodium-to-potassium ratio was calculated by dividing the urinary sodium concentration by potassium concentration.
Statistical analysis
Data were analyzed using GraphPad Prisim® version 5.00 for Windows (GraphPad Software, San Diego, CA). All the data were expressed as means ± SEM. Experimental data were analyzed using repeated measures one-way ANOVA followed by the Bonferroni post-hoc test. The difference between the means was considered significant at the 5% level.
Results
The acute experimental data () demonstrated that the systolic blood pressure, mean arterial blood pressure, and heart rate of all the SHRs groups were higher compared with the WKY group (all p < 0.050). However, in the SHR + NaHS and SHR + Tempol groups, the systolic blood pressure, mean arterial blood pressure, and heart rate were significantly lower than in the SHR (all p < 0.05). There was no significant difference between SHR + NaHS and SHR + Tempol groups in terms of all measured variables (; p > 0.05). Treatment of SHRs with a combination therapy of NaHS and tempol significantly decreased the systolic blood pressure and mean arterial blood pressure in SHR + NaHS + Tempol group to a greater extent compared to the SHR + NaHS and SHR + Tempol groups (all p < 0.05). However, there was no difference in heart rate between that SHR + NaHS + Tempol as compared with the SHR + NaHS and SHR + Tempol groups (; p > 0.05). The renal cortical blood perfusion in all the SHRs groups was significantly lower compared with the WKY group (all p < 0.05). In the SHR groups given NaHS or Tempol treatment alone, the renal cortical blood perfusion was significantly higher compared with the SHR group (both p < 0.05). Renal cortical blood perfusion was not different in the SHR + NaHS and SHR + Tempol groups (p > 0.05). However, the renal cortical blood perfusion in the SHR + NaHS + Tempol group was significantly higher than the SHR + NaHS and the SHR + Tempol groups (; both p < 0.05).
Table 1. Effect of exogenous NaHS, tempol, and a combination of NaHS and tempol on systolic blood pressure (SBP), mean arterial blood pressure (MAP), heart rate (HR), and renal cortical blood perfusion (RCBP) in spontaneously hypertensive rats.
Basal body weight at the start of the treatment period was the same in all groups (). Moreover, the age-dependent body weight gains over the course of treatment were not significantly different between the WKY group and treated or untreated SHRs groups (all p > 0.05). Similarly there was no significant difference in terms of food intake between all experimental groups at each study day (all p > 0.05; ).
Table 2. Effect of exogenous NaHS, tempol, and a combination of NaHS and tempol on body weight, water intake, food intake, urine flow rate (UFR), plasma H2S, and urinary H2S in spontaneously hypertensive rats.
Twenty-four hour water intake and urine flow rate of all the experimental animals were observed on days 0, 14, and 28 and are given in . Water intake and urine flow rate were significantly lower in the SHR group compared with the WKY group on all the 3 d of observation (all p < 0.05; ). However, administration of NaHS or tempol alone or the combination of NaHS and tempol significantly increased water intake on day 28 and urine flow rate on days 14 and 28 compared with the SHR control group (all p < 0.05). Moreover, there was no significant difference between water intake and urine flow rate in the SHR +NaHS + Tempol group compared with the SHR + NaHS or SHR + Tempol groups (both p > 0.05; ).
Plasma and urinary H2S levels were significantly lower in SHR and SHR + Tempol groups on all the 3 d of observation compared with WKY (all p < 0.05; ). With NaHS treatment, plasma and urinary H2S were significantly increased in SHR + NaHS and SHR + NaHS + Tempol groups compared with SHR on day 28 (all p < 0.05; ). Moreover, the SHR + NaHS + Tempol group exhibited a significantly higher level of H2S both in plasma and in urine compared with the SHR + Tempol group on day 28 (both p < 0.05).
Plasma total superoxide dismutase (T-SOD) () level was significantly lower in the SHR, SHR + NaHS, and SHR + Tempol groups compared with the WKY group (all p < 0.05). However, the treatment either with NaHS or Tempol alone significantly increased (both p < 0.05) the T-SOD levels in SHR + NaHS and SHR + Tempol groups compared with the SHR group (120.1 ± 1.4 and 119.6 ± 1.5 vs. 102.3 ± 2.3 U/mL). Moreover, the combination therapy of NaHS and tempol increased the T-SOD level in SHR + NaHS + Tempol group to a significantly higher level compared with that of the SHR + NaHS and SHR + Tempol groups (136.0 ± 2.1 vs. 120.1 ± 1.4 and 119.6 ± 1.5 U/mL) (both p < 0.05).
Figure 1. Oxidant and antioxidant biochemical markers. T-SOD (A), MDA (B), NO (C), and T- AOC (D) of WKY, SHR, SHR + NaHS, SHR + Tempol, and SHR + NaHS + Tempol. The values are mean ± SEM (n = 6). Statistical analysis was done by a repeated measure one-way ANOVA followed by Bonferroni post hoc test. *p < 0.05 versus WKY; †p < 0.05 versus SHR; δp < 0.05 versus SHR + NaHS; #p < 0.05 versus SHR + Tempol. Note: T-SOD, total superoxide dismutase; MDA, malondialdehyde; NO, nitric oxide; T-AOC, total antioxidant capacity.
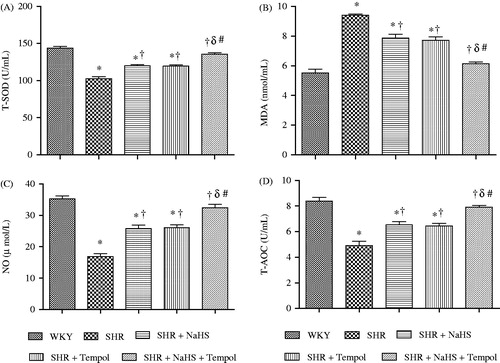
Plasma malondialdehyde (MDA) () concentrations were significantly higher in SHR, SHR + NaHS, and SHR + Tempol groups compared with the WKY group (all p < 0.05). However, treatment with either NaHS or Tempol alone significantly decreased (both p < 0.05) the MDA levels in SHR + NaHS and SHR + Tempol groups compared with the SHR group (7.8 ± 0.2 and 7.7 ± 0.2 vs. 9.4 ± 0.1 nmol/mL). Moreover, the combined treatment significantly decreased the MDA level in the SHR + NaHS +Tempol group compared with the SHR + NaHS and SHR +Tempol groups (6.1 ± 0.1 vs. 7.8 ± 0.2 and 7.7 ± 0.2 nmol/mL) (both p < 0.05).
The plasma level of total antioxidant capacity (T-AOC) and nitric oxide (NO) level () were significantly lower in the SHR, SHR + NaHS and SHR + Tempol than in WKY (all p < 0.05). However, the plasma levels of T-AOC and NO were significantly increased in the SHR + NaHS, SHR + Tempol, and SHR + NaHS + Tempol compared with the SHR group (all p < 0.05). Moreover, T-AOC and NO levels in SHR + NaHS + Tempol groups were significantly higher (all p < 0.05) than either the SHR + NaHS or SHR + Tempol groups (7.9 ± 0.1 vs. 6.5 ± 0.2 and 6.4 ± 0.2 U/mL and 32.3 ± 1.1 vs. 25.7 ± 1.5 and 26.0 ± 1.0 µmol/L), respectively.
The creatinine clearance (Cr.Cl.) and absolute urinary sodium (UNaV) excretion () of the SHR, SHR + NaHS, SHR + Tempol, and SHR + NaHS + Tempol groups remained significantly lower on all the 3 d of observation except for SHR + NaHS + Tempol on day 28 as compared with the WKY group (all p < 0.05). The SHR + NaHS, SHR + Tempol and SHR + NaHS + Tempol groups exhibited increased Cr.Cl. and UNaV on days 14 and 28 compared with the SHR group (all p < 0.05). However, Cr.Cl. and UNaV in the SHR + NaHS + Tempol group were significantly higher (all p < 0.05) than the SHR + NaHS and SHR + Tempol groups on day 28 (6.3 ± 0.2 vs. 5.2 ± 0.1 and 5.1 ± 0.1 mL/min/kg and 0.25 ± 0.01 vs. 0.17 ± 0.00 and 0.17 ± 0.01 mmol/h/kg), respectively. Fractional sodium excretion (FENa) () of all treated and untreated SHRs groups remained significantly lower on days 0 and 14 compared with the WKY group (all p < 0.05). Moreover, the SHR group had a lower FENa on day 28 than that of the WKY group (p < 0.05). The SHR + NaHS, SHR + Tempol and SHR + NaHS + Tempol groups had an increased FENa on day 28 compared to the SHR group (all p < 0.05). FENa of the SHR + NaHS + Tempol group was significantly higher (both p < 0.05) on day 28 compared to the SHR + NaHS or SHR + Tempol groups (0.49 ± 0.01 vs. 0.40 ± 0.01 and 0.41 ± 0.02%).
Figure 2. Cr.Cl. (A), UNaV (B), FENa (C), and Na:K (D) of WKY, SHR, SHR + NaHS, SHR + Tempol, and SHR + NaHS + Tempol. The values are mean ± SEM (n = 6). Statistical analysis was done by a repeated measure one-way ANOVA followed by Bonferroni post hoc test in respective days. *p < 0.05 versus WKY; †p < 0.05 versus SHR; δp < 0.05 versus SHR + NaHS; #p < 0.05 versus SHR + Tempol. Note: Cr.Cl., creatinine clearance; UNaV, absolute urinary sodium excretion; FENa, fractional sodium excretion; Na:K, urinary sodium potassium ratio.
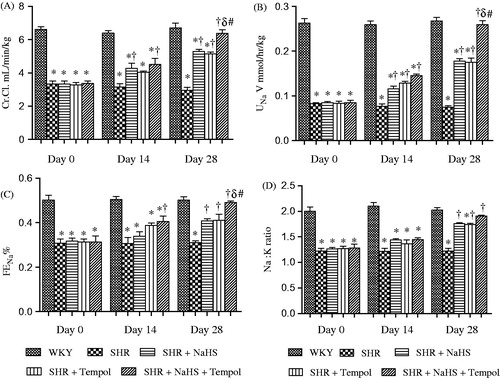
The urinary sodium potassium ratio (Na:K) () of all treated and untreated SHR groups remained significantly lower on days 0 and 14 as compared with the WKY group (all p < 0.05). Moreover, the SHR and SHR + Tempol groups had a lower Na:K on day 28 compared to the WKY group (both p < 0.05). However, it was evident that the SHR + NaHS, SHR + Tempol, and SHR + NaHS + Tempol groups exhibited significantly (all p < 0.05) higher Na:K on day 28 compared with the SHR group (1.76 ± 0.01, 1.74 ± 0.02 and 1.90 ± 0.01 vs. 1.22 ± 0.04). With the NaHS and Tempol combined treatment, no significant differences in Na:K were observed in the SHR + NaHS + Tempol group as compared with either the SHR + NaHS or SHR + Tempol groups (both p > 0.05).
Discussion
This investigation explored the hypothesis that in the SHR, oxidative stress was in part responsible for the genesis and maintenance of the hypertensive state and that there was a significant contribution to the elevated pressure due to a suppression of the H2S/CSE system. It was evident that the SHR groups had higher levels of MDA and lower levels of T-SOD along with decreased plasma total antioxidant capacity and nitric oxide levels as compared with the WKY animals indicative of oxidative stress existing in the hypertensive model.Citation26 Moreover, the SHRs had higher blood pressures and reduced renal excretory function compared with the WKY group. Chronically treating the SHRs with tempol, the superoxide dismutase mimetic, or H2S, a vasodilator, lowered the blood pressure and improved the renal excretory capacity in the SHRs. Interestingly, administration of the two compounds in combination over the 4-week period elicited a larger reduction in blood pressure and a virtual normalization of renal hemodynamic and excretory function in the SHR, comparable with levels found in the normotensive WKY control animals.
The systolic blood pressure and mean arterial blood pressure of the untreated SHR group remained significantly higher (both p < 0.05) compared to WKY groups throughout the 4 weeks of experimental study. The decrease in blood pressure consequent to the administration of the exogenous hydrogen sulfide to the SHRs was attributed to its previously reported vasodilator activity.Citation17,Citation27 In terms of the chronic tempol administration, its antioxidant actionCitation12 is also partly responsible for the reduction in blood pressure which would be in accord with previous reports.Citation13,Citation18 Moreover, the co-treatment with NaHS and tempol resulted in a significantly greater decrease in systolic blood pressure and mean arterial blood pressure compared with the SHR + NaHS and SHR + tempol groups. Taken together, this suggests that the reductions in blood pressure in the SHR following the combination of NaHS and tempol may be influenced by both the antioxidant activity of tempolCitation13 and the up-regulation of the CSE enzyme by exogenous NaHS resulting in increased production of endogenous H2S.Citation18 Supporting this argument, there was a marked increase in plasma H2S levels and urinary H2S excretion. On the basis of the above findings, it would appear that a degree of synergism exists between the actions of H2S and tempol. Oxidative stress and hypertension are usually related to increased sympathetic activityCitation28 which could be responsible for the higher heart rate in the SHR compared with the WKY in the present study. Treating the SHRs with either NaHS or tempol significantly reduced the heart rate compared with SHR which may be related to the inhibitory effects of tempol on sympathetic activity.Citation29 However, whether sympathetic inhibition takes place as a result of exogenous NaHS administration needs further verification.
Superoxide anions (), one of the main ROS, inactivate NO and thereby reduce bioactive NO one result of which may be an acute renal vasoconstriction.Citation30 In the present investigation, it was observed that creatinine clearance, a measure of glomerular filtration rate (GFR) was significantly lower in the SHR group compared with the WKY group. One possible cause of this decreased GFR might be an increase in pre-glomerular arteriolar resistance. The underlying mechanisms for such increased pre-glomerular arteriolar resistance includes the inactivation of endothelium derived NO,Citation31 non-enzymatic generation of vasoconstrictor F-2-isoprostanesCitation32 and/or depletion of the nitric oxide synthase (NOS) cofactor-tetrahydrobiopterin (BH4).Citation33 In the present study, the exogenous H2S donor increased the creatinine clearance after 4 weeks which would be consistent with an earlier report.Citation21 Moreover, a 4-week treatment with tempol significantly increased creatinine clearance in the SHR + tempol treated group suggestive of decreased pre-glomerular arteriolar resistance and is in accord with an earlier study.Citation13 Furthermore, the combination therapy with exogenous NaHS and tempol improved the creatinine clearance more effectively compared with NaHS or tempol alone. Renal cortical blood perfusion was lower in SHRs compared with the WKY indicating an enhanced renal vasoconstriction along the resistance vasculature. NaHS treatment increased the renal cortical blood perfusion which would be compatible with a raised renal arterial blood flow. A similar pattern was observed with tempol treatment in the present study. Moreover, the combined treatment with NaHS and tempol increased the renal cortical blood perfusion to a greater extent than with either compound alone which parallels with the observations in creatinine clearance. It may be argued that such pre-glomerular arteriolar vasodilatation is the result of either decreased superoxide production in renal vessels or due to a reversal of NO bioavailability. In order to clarify this issue, the levels of T-SOD and NO in plasma were measured. Superoxide dismutase is one arm of an important antioxidant defense which dismutases the superoxide to hydrogen peroxide which is then metabolized to water and oxygen by catalases.Citation34 Surprisingly, tempol treatment in the SHRs significantly increased plasma T-SOD and NO levels as did the treatment with NaHS. However, it was found that combined treatment with NaHS and tempol significantly increased the plasma NO and SOD levels to a greater extent than that observed with either treatment alone. One possible explanation can be based on the antioxidant properties of exogenous H2SCitation35 and superoxide dismutase mimetic activity of tempol.Citation12 On the basis of these findings, it can be proposed that the increased superoxide dismutase activity would scavenge the superoxide anions in the vessel walls thereby increasing the bioavailability of NO causing pre-glomerular arteriolar vasodilatation. However, superoxide anions levels were not measured in the present study so the possibility of direct inhibition of superoxide by NaHSCitation36 or tempolCitation37 cannot be ruled out. The increase in creatinine clearance and renal cortical blood perfusion support the view that the combination of NaHS and tempol led to an interaction that resulted in a renal arteriolar vasodilatation and reduced pre-glomerular arteriolar resistance.
An important mechanism by which the kidney contributes to the regulation of long-term blood pressure is altered renal sodium handling to preserve the sodium-retaining properties.Citation38 The present study indicated that UNaV was lower in the SHR group from days 0 to 28 as compared with the WKY. The lower UNaV was primarily due to the reduced GFR causing a lesser amount of sodium to be filtered and excreted. This finding is consistent with the idea that the kidneys of SHRs excrete less sodium as compared with WKY regardless of the high blood pressure of SHRs.Citation39 However, these observed changes imply that the pressure natriuresis relationship is not operating effectively or that there is a resetting in the SHR.Citation40 Several mechanisms have been proposed including increased expression of epithelial sodium channels (ENaC) subunits,Citation3 superoxide anions induced activation of protein Kinase CCitation6 and enhanced Na–K–2Cl co-transporter activityCitation41 in the thick ascending limb of loop of Henle. A decreased FENa as observed in the present investigation may suggest increased sodium reabsorption in the SHRs. In contrast, treating the rats with NaHS or tempol increased UNaV and FENa compared to untreated SHRs which was even greater in the SHR group given the combination of NaHS and tempol. This increase in UNaV and FENa may be explained on the basis of contributions of both exogenous H2SCitation21 and tempolCitation42 to the inhibition of renal Na+/K+–2Cl co-transport mechanism. The accompanying increase in urine flow rate, found in the present study, by NaHS or tempol treatment can be explained on the basis of the observed natriuresis such that the water followed the sodium to augment urine output. It may be argued that such altered renal sodium handling in treated and non-treated SHRs may be due to a difference in dietary intake of sodium but it was evident that there was no significant difference in dietary intake of sodium among all groups. Thus, the increase in UNaV and FENa after combination of NaHS and tempol suggested a potential natriuretic role of these drugs.
Several studies have suggested that hypertension and oxidative stress can be linked through the activation of renin–angiotensin–aldosterone system (RAAS).Citation43 Urinary Na:K reflects renal absorptive function and is inversely proportional to the aldosterone acting on the renal cortical collecting tubules and distal convoluted tubules.Citation44 In the present study, SHRs exhibited a significantly lower Na:K indicative of increased aldosterone activity driven by angiotensin II. This finding is consistent with the idea that SHRs had increased plasma angiotensin II levels despite normal plasma renin activity.Citation45 Thus, it is possible that elevated angiotensin II levels lead to sodium retention in the SHRs through the release of aldosterone. Moreover, increased angiotensin II may, in part, also increase the production of superoxideCitation46 which may lead to sodium retention contributing to the development and maintenance of hypertension. In contrast, treating the SHRs either with exogenous H2S or tempol increased the Na:K. This would suggest that the chronic treatment with exogenous H2S and tempol decreased the aldosterone and angiotensin II activity in SHRs. This may be explained on the basis of the inhibition of angiotensin converting enzyme by exogenous H2SCitation47 and the superoxide scavenging activity of exogenously administered tempol.Citation13,Citation48
It was observed that although both H2S and tempol functions in an independent way, the interaction of the two groups results in a greater response. However, the reductions in blood pressure and increased renal excretion observed in the present study after the combination therapy with exogenous H2S and tempol are neither additive nor potentiating in a true sense as was hypothesized. Therefore, we can assume that there is some interference, that is, at some stage, the compounds are using a common pathway which is rate limiting and preventing a doubling of the responses. However, the above postulation needs further exploration.
Conclusion
In summary, these data demonstrated that enhanced ROS production in SHR contributes to the development and maintenance of hypertension by altering renal vascular and excretory functions. Long-term combined NaHS and tempol therapy is renoprotective and more effective in improving the blood pressure responses and renal excretory functions than treatment with either agent alone.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
The authors fully acknowledge the Fundamental Research Grant no. 203/PFARMASI/6711217 provided by the Ministry of Science Technology and Innovation (MOSTI), Government of Malaysia, for this work.
References
- Vaziri ND, Wang XQ, Ni Z, Kivlighn S, Shahinfar S. Effects of aging and AT-1 receptor blockade on NO synthase expression and renal function in SHR. Biochim Biophys Acta. 2002;1592(2):153–161
- Pinho MJ, Serrao MP, Soares-da-Silva P. High-salt intake and the renal expression of amino acid transporters in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2007;292(5):1452–1463
- Kim SW, Wang W, Kwon T-H, Knepper MA, Frokiaer J, Nielsen S. Increased expression of ENaC subunits and increased apical targeting of AQP2 in the kidneys of spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2005;289(5):957–968
- Welch WJ, Wilcox CS. AT1 receptor antagonist combats oxidative stress and restores nitric oxide signaling in the SHR. Kidney Int. 2001;59(4):1257–1263
- Kitiyakara C, Wilcox S. Antioxidants for hypertension. Curr Opin Nephrol Hypertens. 1998;7(5):531–538
- Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension. 2006;48(3):467–472
- Galle J, Heinloth A, Schwedler S, Wanner C. Effect of HDL and atherogenic lipoproteins on formation of and renin release in juxtaglomerular cells. Kidney Int. 1997;51(1):253–260
- Xu H, Fink GD, Galligan JJ. Nitric oxide-independent effects of tempol on sympathetic nerve activity and blood pressure in DOCA-salt rats. Am J Physiol Heart Circ Physiol. 2002;283(3):885–892
- Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension what is the clinical significance? Hypertension. 2004;44(3):248–252
- Kopkan L, Castillo A, Navar LG, Majid DSA. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2006;290(1):80–86
- Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60(4):418–469
- Patel K, Chen Y, Dennehy K, et al. Acute antihypertensive action of nitroxides in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):37–43
- Welch WJ, Mendonca M, Blau J, et al. Antihypertensive response to prolonged tempol in the spontaneously hypertensive rat. Kidney Int. 2005;68(1):179–187
- Kawada N, Dennehy K, Solis G, et al. TP receptors regulate renal hemodynamics during angiotensin II slow pressor response. Am J Physiol Renal Physiol. 2004;287(4):753–759
- Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206(2):267–277
- Li L, Moore PK. Putative biological roles of hydrogen sulfide in health and disease: a breath of not so fresh air? Trends Pharmacol Sci. 2008;29(2):84–90
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20(21):6008–6016
- Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313(1):22–27
- Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine-lyase. Science. 2008;322(5901):587–590
- Bos EM, Leuvenink HGD, Snijder PM, et al. Hydrogen sulfide-induced hypometabolism prevents renal ischemia/reperfusion injury. J Am Soc Nephrol. 2009;20(9):1901–1905
- Xia M, Chen L, Muh RW, Li PL, Li N. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J Pharmacol Exp Ther. 2009;329(3):1056–1062
- Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;288(1):22–28
- Kopkan L, Majid DSA. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension. 2005;46(4):1026–1031
- Zhang Y, Croft KD, Mori TA, Schyvens CG, McKenzie KUS, Whitworth JA. The antioxidant tempol prevents and partially reverses dexamethasone-induced hypertension in the rat. Am J Hypertens. 2004;17(3):260–265
- Ahmad FD, Sattar MA, Rathore HA, et al. Exogenous hydrogen sulfide (H2S) reduces blood pressure and prevents the progression of diabetic nephropathy in spontaneously hypertensive rats. Ren Fail. 2012;34(2):203–210
- Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15(4):316–328
- Ali MY, Ping CY, Mok Y. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol. 2006;149(6):625–634
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol. 2004;287(2):695–703
- Xu H, Fink GD, Galligan JJ. Tempol lowers blood pressure and sympathetic nerve activity but not vascular in DOCA-salt rats. Hypertension. 2004;43(2):329–334
- Just A, Olson AJM, Whitten CL, Arendshorst WJ. Superoxide mediates acute renal vasoconstriction produced by angiotensin II and catecholamines by a mechanism independent of nitric oxide. Am J Physiol Heart Circ Physiol. 2007;292(1):83–92
- Vaziri ND. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens. 2004;13(1):93–99
- Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-iso prostaglandin F2α. Hypertension. 1999;33(1):424–428
- Hong H-J, Hsiao G, Cheng T-H, Yen M-H. Supplementation with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension. 2001;38(5):1044–1048
- Katusic ZS. Superoxide anion and endothelial regulation of arterial tone. Free Radic Biol Med. 1996;20(3):443–448
- Chai W, Wang Y, Linm JY, et al. Exogenous hydrogen sulfide protects against traumatic hemorrhagic shock via attenuation of oxidative stress. J Surg Res. 2011;176:210–219
- Muzaffar S, Shukla N, Bond M, et al. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J Vasc Res. 2008;45(6):521–528
- Guzik TJ, West NEJ, Pillai R, Taggart DP, Channon KM. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension. 2002;39(6):1088–1094
- Cowley Jr AW, Roman RJ. The role of the kidney in hypertension. JAMA. 1996;275(20):1581–1589
- Beierwaltes WH, Arendshorst WJ, Klemmer PJ. Electrolyte and water balance in young spontaneously hypertensive rats. Hypertension. 1982;4(6):908–915
- Hall JE, Mizelle HL, Hildebrandt DA, Brands MW. Abnormal pressure natriuresis. A cause or a consequence of hypertension? Hypertension. 1990;15(6 Pt 1):547–559
- Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol. 2005;288(5):982–987
- Riazi S, Tiwari S, Sharma N, Rash A, Ecelbarger CM. Abundance of the Na-K-2Cl cotransporter NKCC2 is increased by high-fat feeding in Fischer 344 X Brown Norway (F1) rats. Am J Physiol Renal Physiol. 2009;296(4):762–770
- Cooper SA, Whaley-Connell A, Habibi J, et al. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293(4):2009–2023
- Alexander WD, Branch RA, Levine DF, Hartog M. The urinary sodium: potassium ratio and response to diuretics in resistant oedema. Postgrad Med J. 1977;53(617):117–121
- Bolterman RJ, Manriquez MC, Ruiz MCO, Juncos LA, Romero JC. Effects of captopril on the renin angiotensin system, oxidative stress, and endothelin in normal and hypertensive rats. Hypertension. 2005;46(4):943–947
- Chabrashvili T, Kitiyakara C, Blau J, et al. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):117–124
- Laggner H, Hermann M, Esterbauer H, et al. The novel gaseous vasorelaxant hydrogen sulfide inhibits angiotensin-converting enzyme activity of endothelial cells. J Hypertens. 2007;25(10):2100–2104
- Nishiyama A, Fukui T, Fujisawa Y, et al. Systemic and regional hemodynamic responses to tempol in angiotensin II-infused hypertensive rats. Hypertension. 2001;37(1):77–83

