Abstract
The relationship between pre-transplant Hemoglobin (Hb) concentration and long-term outcome of living-related kidney transplantation is far from well addressed. A retrospective cohort study was conducted by reviewing the medical profile of the patients who received living-related kidney transplantations at our center from January 2006 to January 2013. Patients were divided into two groups: high Hb group (≥10 g/dL) and low Hb group (<10 g/dL). Cox regression model was utilized to analyze the effect of pre-transplant hemoglobin concentration on the patient and graft survival. About 422 patients were of Hb level <10 g/dL (78.30 ± 14.18 g/dL), 280 were >10 g/dL (116.2 ± 14.43 g/dL) (p < 0.001). In a follow-up of 35.34 ± 18.12 months, we did not find any difference in serum creatinine between the two groups. Low Hb concentration is not associated with increased risk of developing DGF (HR = 1.186, 95% CI: 0.53–2.654), acute rejection (HR = 1.338, 95% CI: 0.919–1.947), overall infection (HR = 1.263, 95% CI: 0.847–1.885) nor perioperational infection (HR = 1.019, 95% CI: 0.513–2.026). Though we detected a trend that low Hb level group were of higher incidence of patient death and graft failure, the two groups did not differ significantly (2.38% vs. 0.71%, p = 0.096; and 4.04% vs. 2.14%, p = 0.165, respectively). Cox regression model revealed that pre-transplant Hb level <10 g/dL was independent of increased overall mortality (HR = 3.379; 95% CI: 0.706–17.172) and increased death censored allograft failure risk (HR = 1.556; 95% CI: 0.595–4.069). Pre-transplant Hb concentration <10 g/dL is independent of poor long-term outcome of living-related kidney transplantation.
Introduction
Anemia is universal in patients with chronic kidney disease (CKD), attributed to several factors including impaired endocrine function in production of erythropoietin, iron, folate or vitamin B12 deficiency, gastrointestinal blood loss, shortened red blood cell survival, and less frequently, by disorders such as hemolytic uremic syndrome or aplastic anemia.Citation1,Citation2 Of them, decreased erythropoietin synthesis is the most important and specific etiology causing CKD-associated anemia. After the kidney transplantation, anemia remains a common complication with a prevalence ranges from 20% to 60% at 1 year and >30% during the follow up.Citation3–6 There is a growing body of literature documented anemia in the post-transplant period, demonstrating that anemic condition associated with a reduced graft and patient survival.Citation7–9 However, little is known about the relationship between pre-transplant hemoglobin (Hb) level and long-term outcome in kidney recipients. Results from retrospective studies with small patient population indicated higher pre-transplant Hb conferred comparable patient and graft outcome, but a better graft function.Citation10,Citation11 Another study with the attempt to correct pre-transplant anemia with recombinant human erythropoietin (rHuEPO) showed that rHuEPO-hyporesponsive patient suffered higher mortality and higher incidence of graft failure at 5 years,Citation12 suggesting an elevation in pre-transplant Hb may improve long-term outcome. Nevertheless, Muirhead et al.Citation13 found that pre-transplantation Hb concentration did not significantly affect DGF, graft loss, or vascular thrombosis in kidney recipients.
Given the uncertainty of whether correcting anemic condition before transplantation will result in better long-term outcome, we reported our single-center experience, seeking to evaluate the usefulness of correcting anemia by reaching a targeting pre-transplant Hb level.
Materials and methods
Patients
Between 1 January 2006 and 30 June 2012, a total of 702 living-related kidney transplants were performed in our center. The demographic and clinical data were obtained by reviewing patients’ profile. As the previous studies revealed that patients with pre-transplant Hb level <10 g/dL experienced comparable short-term outcome but poorer renal function,Citation11,Citation12 patients were divided into two groups according to the pre-transplant Hb concentration: <10 g/dL and ≥10 g/dL. During the follow up period, the Hb concentration and serum creatinine were collected at months 3 and 6 after transplant and every 6 months thereafter.
Immunosuppressive regimen
Induction therapy by rabbit anti-human thymocyte immunoglobulin (1.0–1.5 mg kg−1 d−1 for 3–5 days) or anti-CD25 monoclonal antibody (1.0–1.5 mg kg−1 right before the surgery and 10–14 days thereafter) was instituted according to the pre-transplantation evaluation of recipients. Maintenance immunosuppression therapy consisted of a calcineurin inhibitor [CNI; cyclosporine A (CsA) or tacrolimus (TAC)] in combination with mycophenolate mofetil (MMF) and prednisone. CsA or TAC was initiated at the 2nd day post-transplant with a fixed dosage of CsA 100 mg or TAC 2 mg, twice a day. MMF was given 1 day before the transplant with a dosage of 2.0 g d−1. The dosage was adjusted according to the blood drug concentration, with a trough level for TAC of 5–8 ng mL−1, CsA of 75–150 ng mL−1 and MMF area under the curve 30–60 mg h/L−1. Methylprednisolone was given on the day of surgery of 7 mg kg−1 intravenously for 3 days, and then given as prednisone of 60 mg d−1 and tapering 10 mg d−1, maintained with 5–10 mg d−1 thereafter.
Primary outcomes of the study were patient and graft survival, secondary outcomes were the incidence of DGF, the rejection free interval during the post-transplant period and the infection. Death censored graft survival is death with a functioning graft. DGF was defined as need for dialysis after transplantation. Rejection was diagnosed on the basis of an increase in serum creatinine, confirmed by examination of a biopsy sample in needed and was treated primarily with bolus doses of methylprednisolone and, in cases of refractory rejection, with antithymocyte globulin. Infection was defined as any infection the recipients get, including wound infection, pulmonary, urinary tract and skin infection. For further analysis, we also analyze the incidence of perioperational infection (<1 month after surgery).
Statistical analysis
Demographic characteristics were compared using the chi-squared test for category variables and means ± SE for continuous variables. Comparisons were made between groups in both primary and secondary outcomes. Quantitative variables were compared by Student’s t test or the Wilcoxon test, when appropriate. Logistic regression was applied to estimate the relationship between DGF, infection and pre-transplant Hb. The first acute rejection episode, patient and kidney graft survivals were performed according to the Kaplan–Meier method supplemented by hazard ratio (HR) calculations based on Cox proportional models. Statistical significance was indicated by a two-tailed p < 0.05 for all analysis. Data analysis was performed with SPSS 17.0 software (SPSS Inc., Chicago, IL).
Results
About 422 patients (60%) were of Hb level <10 g/dL (mean Hb, 78.30 ± 14.18 g/dL), 280 (40%) were >10 g/dL (116.2 ± 14.43 g/dL, ). There is no difference in donor-related characteristics. Other than Hb level, all demographic data regarding recipients are of no significant discrepancy, except the higher Hb level group receiving dialysis of a longer period, lower rate of erythropoiesis-stimulating agent use and iron supplementation. Both groups are comparable in HLA mismatch, panel reactive antibody (PRA) screening and induction therapy. Majority use TAC and MMF as their maintenance immunosuppression regime, 62.32% in low Hb group and 71.07% in high Hb group. During the follow up, 47 recipients receiving CsA as primary immunosuppressant in low Hb group converted to TAC, and 15 in the high Hb group. Almost half conversion was because of acute rejection or creeping serum creatinine and the left was attributed to complications, such as hairy or gingival hyperplasia.
Table 1. Demographic data of included patients.
Mean follow up period was 35.34 ± 18.12 months (ranged from 1 to 84 months). At 3 months, the Hb concentration of low Hb group reached 12.77 and 13.35 g/dL for high Hb group. Significant difference in Hb concentration between the two groups persists until 54 months later. However, no difference was detected in serum creatinine throughout the follow up period.
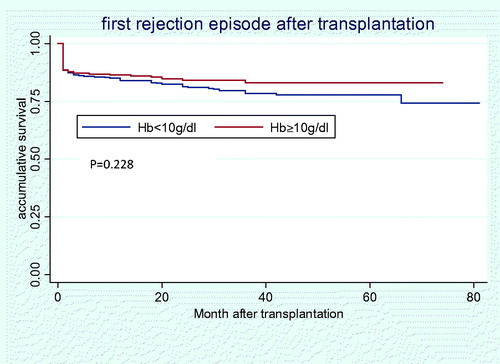
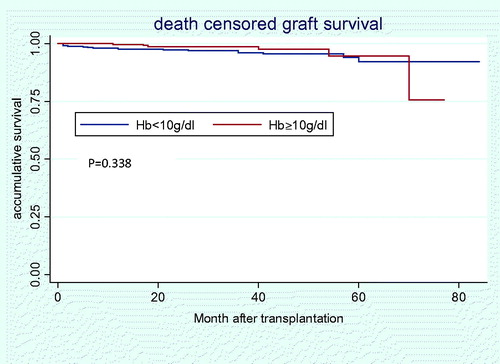
About 12 (1.71%) deaths occurred. Among them, nine were because of lung infection, two cardiovascular events and one intestine obstruction. Furthermore, there were 23 graft failures. Though there is a trend that low Hb group is of higher incidence of patient death and graft failure, the two groups did not differ significantly (2.38% vs. 0.71%, p = 0.096; and 4.04% vs. 2.14%, p = 0.165, respectively). Cox regression model found that pre-transplant Hb<10 g/dL was not associated with significantly increased overall mortality (HR = 3.379; 95% CI: 0.706–17.172) () and increased death censored allograft failure (HR = 1.556; 95% CI: 0.595–4.069) ().
Figure 1. Cumulative patient survival in Hb < 10 g/dL versus Hb ≥ 10 g/dL group after transplantation.
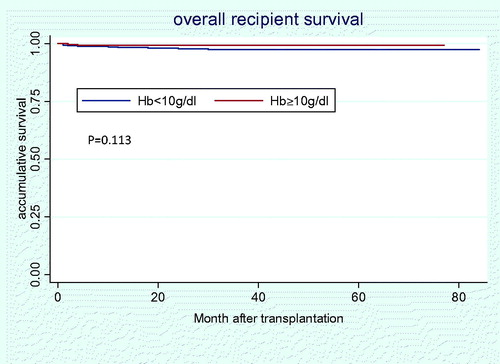
Figure 2. Death censored renal allograft survival in Hb < 10 g/dL versus Hb ≥ 10 g/dL group after transplantation.
During the follow up, 27 (3.85%) recipients developed DGF, 16 in low Hb group, 11 in high Hb group, with similar incidences (3.8% vs. 3.91%, p = 0.939). Balancing other confounding factors, recipients with lower Hb level were of higher risk in developing DGF, but did not reach the level of significance (HR = 1.186, 95% CI: 0.53–2.654). About 127 (18.09%) recipients developed acute rejection, 84 (19.91%) in low Hb group, 43 (15.36%) in high Hb group (p = 0.15). About 81 rejection episodes (63.78%) occurred in the first month, and the average time since the operation was 6.46 ± 11.07 months. We did not observe any protective effect exerted by higher Hb level in acute rejection avoidance (HR = 1.338, 95% CI: 0.919–1.947) (). With respect to overall infection, 97 (23.0%) recipients got infection in low Hb group during the follow up, and 53 (18.9%) in high Hb group (p = 0.308). Additionally, there was 26 perioperational infection in low Hb group and 16 in high Hb group (p = 0.887). No increased risk for low Hb level was found either in overall infection (HR = 1.263, 95% CI: 0.847–1.885) or in perioperational infection (HR = 1.019, 95% CI: 0.513–2.026).
Discussion
The overall prevalence of CKD-associated anemia is ∼50%, and positively correlated to the advanced disease stage. It is estimated that three-quarters of CKD patients starting dialysis suffer from anemia.Citation14 CKD-associated anemia increases morbidity and mortality from cardiovascular complications, including angina, left ventricular hypertrophy and worsening heart failure.Citation15 In addition, anemia is an independent predictor of death in stable coronary artery disease patients with CKD.Citation16 In patients received kidney transplantation, anemic conditions prevail, even years later.Citation3–7 Post-transplant anemia has been proved to associate with greater risk of allograft loss,Citation8,Citation9,Citation17 reduced patient survival.Citation5,Citation7,Citation18 Furthermore, correction of post-transplant anemia will improve the long-term outcome by reducing progression of chronic allograft nephropathy.Citation19
In the present study, pre-transplant Hb concentration is independent of poor allograft and patient survival, an observation in agreement with the findings of Na,Citation11 who divided recipients into two groups: high Hb group (≥100 g/L) and low Hb group (<100 g/L), and followed up for 1 year, revealing similar incidence of acute rejection (7.9% vs. 11.4%, p > 0.05); DGF (11/68 vs. 7/56), comparable patients survival (98.7% vs. 96.8%) and graft survival (98.7% vs. 96.8%).Citation11 Another two studies corroborated the aforementioned observations.Citation12,Citation20 Additionally, the latter two studies also showed that the significant difference in Hb concentration between the two groups persist at 1-year post-transplant. This is in accordance to our results, and comparable Hb level were not attained until 54 months after transplantation.
A report by Molnar et al.,Citation7 who, in a prospective cohort study, found that anemia at baseline significantly predicted mortality and graft failure over 4-year follow-up. Another retrospective studies demonstrated that post-transplant anemia at 12 mo (defined as Hb < 12 g/dL) was associated with decreased patient and graft survival.Citation5 Gheith et al.Citation9 found that anemic condition (Hb < 13 g/dL in males and <12 g/dL in females) at 6 months post-transplant was associated with poorer graft survival, with 93.9% versus 84.8% at 10 years. Moreover, recipients of post-transplant anemia at 3 months (Hb <11 g/dL) were found of significant higher risk of overall mortality (HR = 3.18) and graft loss (HR = 2.67).Citation21 Based on these results, we can extrapolate that only the anemic duration the allograft experienced would greatly affect the fate of the allograft, rather than the pre-transplant anemia. If the anemic condition can be reversed promptly, as our results indicated that both groups got anemic condition corrected at 3 months, the negative affect it exerted might be negligible.
While anemia in CKD can result from multiple mechanisms, decreased erythropoietin synthesis is the predominant one, attributing to tubulointerstitial fibrosis, which compromises renal erythropoietin synthetic capacity. After transplantation, with restoration of kidney filtration function and erythropoietin synthesis, most of the anemic condition could be corrected. As is shown in our results, the recipients of low Hb group get an Hb increment of 5 and 2 g/dL in high Hb group at 3 months. However, there is 28 patients (6.65%) in low Hb group did not reach a Hb concentration of >10 g/dL at 3 months. Even all receiving erythropoiesis-stimulating agents and iron supplementation, indicating a hyporesponsiveness of erythropoietin stimulation or the impaired bone marrow hematopoietic function. As suggested by Campise et al.,Citation12 rHuEPO-hyporesponsive patients suffered a significant higher incidence of graft failure comparing to rHuEPO responders (50% vs. 41.7%), and a higher risk of graft failure (41.7% vs. 32%). That these population whose Hb concentration do not improve months after the restoration of kidney function need further investigation, and alternative treatment other than erythropoiesis-stimulating agents may required.
Our study has several limitations, and the results should be interpreted with caution. One important limitation is in the intrinsic design of the study. Our data, in fact, were collected retrospectively, similar with other studies in this area. A second important limitation is that, the Hb cut off value is arbitrary, because no consensus has reached on the management of pre-transplant anemia. Further investigation on this subject is needed to determine the optimal pre-transplantation Hb concentration.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
This project was supported by the National Natural Science Foundation of China (Grant No. 30872579), [email protected]
References
- Eschbach JW, Adamson JW. Anemia of end-stage renal disease (ESRD). Kidney Int. 1985;28:1–5
- Turkowski-Duhem A, Kamar N, Cointault O, et al. Predictive factors of anemia within the first year post renal transplant. Transplantation. 2005;80(7):903–909
- Al-Khoury S, Shah N, Afzali B, Covic A, Taylor J, Goldsmith D. Posttransplantation anemia in adult renal allograft recipients: prevalence and predictors. Transplantation. 2006;81(8):1112–1118
- Afzali B, Al-Khoury S, Shah N, Mikhail A, Covic A, Goldsmith D. Anemia after renal transplantation. Am J Kidney Dis. 2006;48(4):519–536
- Imoagene-Oyedeji AE, Rosas SE, Doyle AM, Goral S, Bloom RD. Posttransplantation anemia at 12 months in kidney recipients treated with mycophenolate mofetil: risk factors and implications for mortality. J Am Soc Nephrol. 2006;17(11):3240–3247
- Yorgin PD, Scandling JD, Belson A, Sanchez J, Alexander SR, Andreoni KA. Late post-transplant anemia in adult renal transplant recipients. An under-recognized problem? Am J Transplant. 2002;2(5):429–435
- Molnar MZ, Czira M, Ambrus C, et al. Anemia is associated with mortality in kidney transplanted patients – a prospective cohort study. Am J Transplant. 2007;7(4):818–824
- Ott U, Busch M, Steiner T, Wolf G. Anemia after renal transplantation: an underestimated problem. Transplant Proc. 2008;40:3481–3484
- Gheith O, Wafa E, Hassan N, et al. Does posttransplant anemia at 6 months affect long-term outcome of living donor kidney transplantation? A single center experience. Ghoneim MA Clin Exp Nephrol. 2009;13(4):361–366
- Abou-Jaoude MM, Nawfal N, Najm R, Honeidi M, Shaheen J, Almawi WY. Effect of pretransplantation of hemoglobin blood concentration on renal allograft survival and function. Transplant Proc. 2010;42(3):760–762
- Na N, Hong LQ, Miao B, Hua XF, Huang ZY. Effect of pre-transplantation hemoglobin concentration on prognosis of renal transplant recipients. Chin Med J (Engl). 2011;124(8):1213–1216
- Campise M, Mikhail A, Quaschning T, Snyder J, Collins A. Impact of pre-transplant anemia correction and erythropoietin resistance on long-term graft survival. Nephrol Dial Transplant. 2005;20(Suppl 8):viii8--viii12
- Muirhead N. Erythropoietin and renal transplantation. Kidney Int. 1999;69:s86–s92
- McClellan W, Aronoff SL, Bolton WK, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20:1501–1510
- Besarab A, Levin A. Defining a renal anemia management period. Am J Kidney Dis. 2000;36:S13–S23
- Muzzarelli S, Pfisterer M. Anemia as independent predictor of major events in elderly patients with chronic angina. Am Heart J. 2006;152:991–996
- Winkelmayer WC, Chandraker A, Alan Brookhart M, Kramar R, Sunder-Plassmann G. A prospective study of anemia and long-term outcomes in kidney transplant recipients. Nephrol Dial Transplant. 2006;21(12):3559–3566
- Kamar N, Rostaing L. Negative impact of one-year anemia on long-term patient and graft survival in kidney transplant patients receiving calcineurin inhibitors and mycophenolate mofetil. Transplantation. 2008;85(8):1120–1124
- Choukroun G, Kamar N, Dussol B, et al. Correction of post-kidney transplant anemia reduces progression of allograft nephropathy. J Am Soc Nephrol. 2012;23(2):360–368
- Glicklich D, Kutcher R, Rosenblatt R, et al. Time-related increase in hematocrit on chronic hemodialysis: uncertain role of renal cysts. Am J Kidney Dis. 1990;15(1):46–54
- Chhabra D, Grafals M, Skaro AI, Parker M, Gallon L. Impact of anemia after renal transplantation on patient and graft survival and on rate of acute rejection. Clin J Am Soc Nephrol. 2008;3(4):1168–1174