Abstract
Objectives: This study estimated plasma levels of interleukin IL-1β, IL-6, tumour necrosis factor-α (TNF-α), interferon-γ (INF-γ) in chronic kidney disease (CKD) patients with a single odontogenic pathology. Material and methods: Forty-nine selected adult CKD patients with single odontogenic pathology based on clinical and X-ray examination: patients after proper root canal treatment, without periapical lesions (n = 12), with pulp necrosis (n = 7), with asymptomatic periapical lesions (n = 22), with periodontal disease (n = 8), and 14 with healthy teeth were enrolled. Patients with coexisting different dental pathologies and the evidence of other infection were excluded. In all patients plasma concentrations of CRP, IL-1β, IL-6, TNF-α, and INF-γ were measured. Results: Patients with periodontitis were characterized by increased concentrations of IL-6 and TNF-α. Those with pulp necrosis had significantly more frequently serum CRP level over 2 mg/L and presented significantly elevated IL-6, but decreased TNF-α concentration than in the subjects with healthy teeth. In patients with periapical lesions and patients after root canal therapy, the concentrations of cytokines did not indicate for the systemic inflammation. Conclusions: Periodontitis and pulp necrosis are important sources of systemic microinflammation in CKD patients. Plasma concentrations of IL-6 and TNF-α appear to be more sensitive markers of odontogenic inflammation in CKD patients than CRP.
Introduction
Recently, it was found that uremic patients have more dental problems than healthy age- and gender-matched controls, and that they may develop their dental problems even before initiation of dialysis therapy.Citation1 Dialysis patients presented more severe periodontitis (i.e., periodontal loss of attachment) and a higher rate of periapical lesions occurrence. These finding are in line with previous results of an epidemiologic cross-sectional ARIC (Atherosclerosis Risk In Communities) study performed in 5537 middle age women and men.Citation2 In this study, an initial or severe periodontal disease was associated with at least two times higher prevalence of chronic kidney disease (CKD), defined as estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m2. In American–Indian population with type 2 diabetes, a moderate and severe periodontitis and edentulousness predicted the development of macroalbuminuria and end-stage renal disease (ESRD) during follow-up of up to 22 years.Citation3 Severe periodontitis occurrence has doubled the risk of macroalbuminuria development and the risk of ESRD was 3.5 times as high after the adjustment for HbA1c.
In addition, the advanced periodontal disease increases the cardiovascular risk. In the NHANES III cohort (5564 individuals), a severe periodontal disease was associated with almost 4-fold higher incidence of myocardial infarction compared with patients without periodontal disease, after adjustment for age, gender, race, socioeconomic status, smoking, blood pressure, body mass index, and serum cholesterol.Citation4 Thus, the periodontal and periapical inflammation seems to be an important source of systemic inflammation, partially responsible for the progression of CKD and a burden of atherosclerotic complications in dialysis patients.
On the other hand, an increased local release of interleukin 1β (IL-1β), interleukin 1 receptor antagonist (IL-1RA), and interleukin-6 (IL-6) was found in periapical lesions of endodontic origin.Citation5–7 It is unknown whether this local production of cytokines could significantly increase the levels of circulating cytokines and enhance the liver C-reactive protein (CRP) production, like in patients with periodontitis.Citation8 It is of note that periodontal and periapical inflammations are potentially treatable entities. Treatment of periodontitis by tooth extractionCitation9 or other mechanical procedures and locally administered antibioticsCitation10 lowers serum concentration of CRP and perhaps other markers of systemic inflammatory reaction.
Therefore, we estimated plasma levels of IL-1β, IL-6, tumour necrosis factor α (TNF-α) and interferon γ (INF-γ) by applying highly sensitive enzyme-linked immunoabsorbent assays in a group of CKD patients with only single odontogenic pathology.
Materials and methods
Patients
Crude recruitment of adult patients with chronic kidney disease was made among hypertensive patients of the Department of Nephrology, Endocrinology and Metabolic Diseases at Medical University of Silesia in Katowice. All patients underwent routine diagnostics, including laboratory tests: blood count, erythrocyte sedimentation rate, serum concentration of creatinine, electrolytes, glucose, total cholesterol as well as LDL and HDL fractions, CRP, urinalysis, 24-h protein excretion, ultrasound examination of abdomen and carotid vessels and echocardiography, ORL examination and, in addition, gynecological examination in women. The inclusion criteria comprise eGFR below 90 mL/min/1.73 m2 or permanent proteinuria over 0.3 g/L or permanent erythrocyturia. The exclusion criteria included BMI over 40 kg/m2, the presence of acute or chronic infections of other than odontogenic origin, the presence of systemic disease, cancer disease or liver cirrhosis.
Patients were then referred for odontogenic evaluation to the Outpatient Clinic at the Department of Craniomaxillofacial Surgery. Throat smears were taken, and paranasal sinus X-rays were obtained to exclude the patients with local pathologies other than odontogenic infection foci. Based on clinical examination and dental pantomogram, 63 patients (30 women and 33 men, aged 17–69 years) with only single odontogenic infection from more than 200 eligible were finally enrolled into this study. Patients with any combine dental pathology were excluded. The patient’s characteristics are summarized in . The study protocol was approved by Local Bioethics Committee (KNW-6501-32/08) and informed consent was obtained from each patient.
Table 1. Characteristics of 63 hypertensive patients with chronic kidney disease and odontogenic infection.
The group of patients was divided into four subgroups, depending on the type of dental pathology. The first subgroup comprised 12 patients after proper root canal treatment, with no periapical lesions (or other dental pathologies). The second subgroup consisted of seven patients with pulp necrosis, but without periapical lesions; the third subgroup included 22 patients with asymptomatic periapical lesions, and the fourth subgroup comprised of eight patients with periodontal disease manifested as horizontal or vertical periodontal bone loss. The evaluation was based on the Gingival Index (GI, scores 2 and 3) proposed by Silness and Löe,Citation11 and the Russell's Periodontal Index (scores 6 and 8).Citation12 Fourteen CKD patients with healthy dentition and a physiological throat culture served as the reference subgroup (R).
Sonographic examination
Echocardiography and the measurement of intima-media thickness (IMT) of both common carotid arteries were performed by one experienced sonographer with Acuson Aspen machine (Mountain View, CA), equipped with 2.5–4.0 MHz micro-convex-array and 7.0–10.0 MHz linear transducers. The mean value of measurements of both common carotid arteries was calculated for each study participant. Standard echocardiography parameters were measured and calculated, including left ventricular ejection fraction (EF) and left ventricular mass based on Devereux formula.
Laboratory measurements
Venous blood samples were withdrawn in all patients at the morning after overnight fasting for the assessment of plasma concentrations of CRP, IL-6, IL-1β, and TNF-α. Blood samples were collected for EDTA, centrifuged at 5000 rpm for 10 min. The obtained plasma was stored frozen at −70 °C until assessment.
Serum CRP concentration was measured by nephelometric method using “CardioPhase hsCRP” kits from Siemens Healthcare Diagnostics (Deerfield, IL) after 200 times sample dilution using a Dade Behring nephelometer (Dade Behring Inc., Deerfield, IL). The lowest detected plasma CRP concentration was 2 mg/L.
IL-1β, IL-6, and TNF-α concentrations were determined with a highly sensitive ELISA Quantikine kits (R&D Systems, Minneapolis, MN). The lower limits of their sensitivity were 0.13, 0.30, and 0.10 pg/mL, respectively. INF-γ concentration was estimated by high sensitivity ELISA kit (Diaclone SAS, Besançon, France) with the lower limit of sensitivity of 0.20 pg/mL.
Statistics
Statistical analysis was performed using STATISTICA 8.0 software package (StatSoft Polska, Cracow, Poland). Data are presented as mean ± SD or mean and 95% confidence interval (in brackets). The Mann–Whitney U test was used to determine the differences between analyzed subgroups. Receiver operator curve (ROC) analyses were used for the estimation of IL-6 and TNF-α cut-off values typical for pulp necrosis and periodontitis. The results were considered as significant with p value of less than 0.05.
Results
Patients’ characteristics
The demographic characteristics of study participants are given in . The mean duration of arterial hypertension was 5.9 ± 6.8 years. About 52.4% of study patients were receiving at least three antihypertensive drugs, 43.1% had advanced hypertensive retinopathy (II° or more) and 33.3% had eGFR below 60 mL/min/1.73 m2. About 42.5% of patients presented significant proteinuria (> 1.0 g/24 h) and 17.5% of patients were diagnosed with hypertensive nephropathy. In the majority of patients (52.4%), the diagnosis of the kidney disease was not established ().
The patients without odontogenic infection foci (reference subgroup) were younger, and had the less impaired kidney excretory function. The subgroup of patients with periodontitis was the oldest and had thicker IMT than the other ones (). There were no significant differences in echocardiography parameters between study subgroups.
Table 2. Lipid profiles, intima-media thickness (IMT), and echocardiography data in hypertensive patients with odontogenic infection foci and chronic kidney disease.
Microbiological examination
The affected teeth were extracted in 17 out of 22 patients with periapical lesions, in 6 out of 8 patients with periodontitis, in 5 out of 8 patients with pulp necrosis, and in 5 out of 12 patients after root canal therapy without periapical lesions. The microbiological examination revealed the growth of Streptococcus viridians in all but one alveolar smears. Neisseria spp. was found in 47% of smears in participants with periapical lesions, 67% of patients with periodontitis, 40% of those with pulp necrosis, and in 60% of patients after root canal therapy. The other bacteria were identified less often, including: Hemophilus spp. (n = 7), Enterococcus faecalis (n = 1), Corynebacterium spp. (n = 1) Citrobacter youngae (n = 1), and yeasts Candida albicans (n = 3).
There were no statistical differences in alveolar flora of the analyzed subgroups and the subgroup with healthy dentition. Streptococcus viridians were found in all participants, Neisseria spp. in 56% and Hemophilus spp. in 11.1%.
Markers of inflammation
We did not observe significant differences in mean concentration of CRP between all study subgroups (), however in the majority of patients with pulp necrosis CRP concentration was above the lowest level of sensitivity of the applied method of detection (>2 mg/L). Patients with pulp necrosis presented significantly higher plasma IL-6 concentrations, while significantly lower TNF-α levels than in the reference subgroup ( and and ). Patients with periodontitis were characterized by increased concentrations of both IL-6 and TNF-α ( and and ). In patients with periapical lesions and after root canal therapy any of the analyzed parameters did not indicate the presence of the systemic inflammation.
Figure 1. Plasma concentration of tumour necrosis factor α in hypertensive patients with odontogenic infection foci and chronic kidney disease: I—patients after proper root canal therapy, with no periapical lesions, II—patients with pulp necrosis, III—patients with asymptomatic periapical lesions, IV—patients with periodontal disease, and reference subgroup with health teeth (statistical significance vs. reference subgroup *p = 0.02; **p = 0.001).
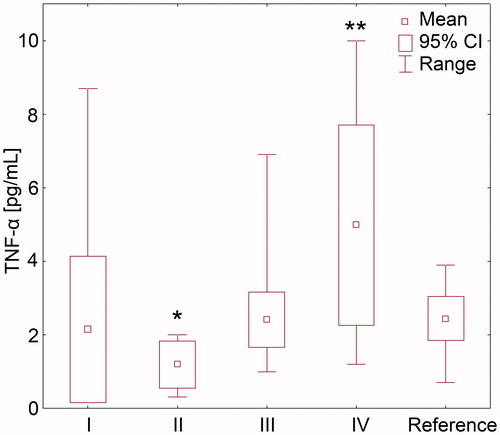
Figure 2. Plasma concentration of interleukin 6 in hypertensive patients with odontogenic infection foci and chronic kidney disease: I—patients after proper root canal therapy, with no periapical lesions, II—patients with pulp necrosis, III—patients with asymptomatic periapical lesions, IV—patients with periodontal disease, and reference subgroup with health teeth (statistical significance vs. reference subgroup **p = 0.001).
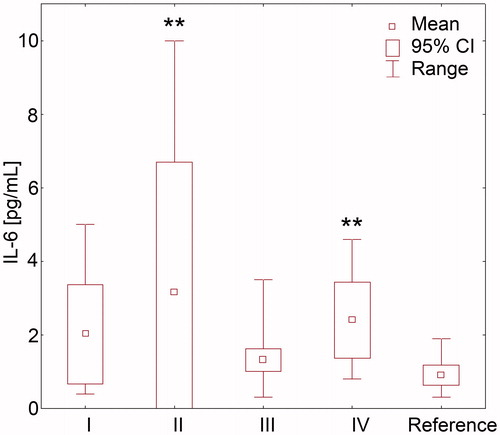
Table 3. Plasma concentration of inflammatory markers in hypertensive patients with odontogenic infection foci and chronic kidney disease.
We applied ROC analysis for discrimination of cut-off values for IL-6 and TNF-α indicating the pulp necrosis or periodontitis occurrence. Plasma IL-6 greater than 1 pg/mL and TNF-α less than or equal to 2 pg/mL enable to discriminate pulp necrosis presence with 100% sensitivity and 77% specificity. The cut-off values for patients with periodontitis were as followed: >1.3 pg/mL for IL-6 (87% sensitivity and 85% specificity) and >3.1 pg/mL for TNF-α (62% sensitivity and 85% specificity).
Discussion
As it was shown in this study, only some of odontogenic inflammatory diseases, namely pulp necrosis and periodontitis, are able to significantly increase the concentration of circulating cytokines. Serum IL-6 and TNF-α concentrations were shown to be superior to CRP as markers of odontogenic inflammatory status in CKD patients. The other markers (IL-1β and INF-γ) were not useful for this purpose.
CKD is associated with an increased inflammatory state.Citation13 Dental pathology is postulated as a significant and common source of chronic inflammation in these patients. Periodontitis is the most manifested inflammatory odontogenic state of increasing prevalence with CKD progression.Citation14,Citation15 It is a destructive inflammatory disease of the supporting tissue of teeth, caused by chronic infection with specific Gram-negative bacteria species, i.e., Porphyromonas gingivalis, Prevotella intermedia, Tannaerella synthesis, and Aggregatibacter actinomycetemcomitans. Subgingival biofilm constitutes a reservoir of Gram-negative bacteria and their products (i.e., lipopolysaccharides), and through periodontium acts as a source of locally produced inflammatory mediators. The total surface area of inflamed periodontal tissue can reach up to 20 cm2, depending upon the number of affected teeth and severity of the disease.Citation16 Several previously published studiesCitation17–25 showed the significantly increased serum concentrations of CRP and cytokines, i.e., IL-6 and TNF-α in patients with periodontitis. On the other hand, our results showing the lack of elevation in IL-1β and IFN-γ serum concentrations are in line with the findings of Górska et al. They reported a high concentrations of IL-1β and IFN-γ in collected tissue supernatants, but not in the serum of patients with advanced periodontitis.Citation26
The presence of periodontal pathogens (Porphyromonas gingivalis, Prevotella intermedia, Campylobacter recta and Bacteroides forsythus) in subgingival plaque samples was associated with higher CRP levels.Citation18 A meta-analysis of 10 cross-sectional studies estimated a mean difference in CRP level between periodontitis patients and controls for 1.56 mg/L.Citation27 It has been shown that CRP level is increasing along with the severity of periodontitis.Citation17,Citation18 Increased CRP level has been also found in hemodialysis patients with periodontitis,Citation28 especially those with destructive periodontal diseases and elevated levels of IgG antibody against Porphyromonas gingivalis.Citation29 Pussinen et al. showed that high antibody titers to Porphyromonas gingivalis was associated with 2–3 times higher incidence of coronary artery disease.Citation30
However, we did not observe increased CRP serum concentration in patients with dental pathology, even in those with periodontitis. This is not a unique observation. Marcaccini et al. found increased IL-6, but not CRP serum levels in a group of non-smoking, otherwise healthy patients with periodontitis.Citation31 It is worth to stress that in several previous studies the CRP level in patients with periodontal pathology was below 3 mg/L. Loos et al.Citation17 found only slightly increased CRP level in patients with generalized periodontitis (1.45 mg/L) and localized periodontitis (1.3 mg/L) than in healthy controls (0.9 mg/L). Higher CRP values were observed in patients with aggressive, rapidly progressing periodontitis (9 mg/L vs. 2 mg/L in controls).Citation19 The method of CRP assessment, used in this study, allows detecting plasma CRP concentration ≥2 mg/L. This also could potentially explain the lack of significant differences in CRP levels between study subgroups.
In our study, the subgroup of patient with periodontitis was characterized by greater IMT value of the carotid artery, a surrogate marker of atherosclerosis. However, as IMT is known to increase with age and patients with periodontitis were older in this study that makes the interpretation of this finding difficult. It is of note, that we were practically unable to find an appropriate number of reference patients (i.e., without any identifiable dental pathology) in the age group >35 years, which is one of the limitations of our study. Nevertheless, Beck et al. reported that severe periodontitis was associated with greater IMT value (>1 mm), also after adjustment for other confounding factors for atherosclerosis like age, male gender, serum level of LDL, HDL, and triglycerides, diabetes, hypertension, smoking status, and standardized waist-to-hip ratio.Citation32 Periapical inflammatory lesions are a frequent pathology and, in most cases, a consequence of dental caries with subsequent pulp necrosis. It triggers an inflammatory process with infiltration of monocytes and macrophages, which are releasing a wide range of cytokines including IL-6, IL-1β, IL-1 receptor antagonist (IL-1RA), and TNF-α.Citation33 Barkhordar et al. demonstrated a high concentrations of IL-1β in periapical lesions and of IL-6 in inflamed pulp and periapical lesions of endodontic origin.Citation5,Citation6 In the healthy pulp, their concentrations were either undetectable or thousand times lower. Slightly higher IL-6 and TNF-α concentrations were found in symptomatic than asymptomatic periapical lesions.Citation34 Danin et al.Citation35 was analyzed concentrations of IL-1RA, IL-6, and transforming growth factor β1 (TGF-β1) in periapical tissue extracts from 22 patients with root-filled teeth with periapical lesions. Only IL-1RA concentrations were significantly higher in patients untreated with antibiotics. This may suggest that IL-1RA could be the best marker of periapical inflammation. Until now, it was unknown if this local inflammation may have an impact on systemic circulating cytokines levels. Recently, the progress in analytic techniques and introduction of highly sensitive ELISA kits enable the detection of much lower cytokine concentrations than previously. However, even applying these methods we were unable to demonstrate the influence of periapical lesions on circulating levels of analyzed cytokines. Taking into account that similar serum cytokine levels were found in patients after root canal therapy both with and without periapical lesions, we may assume that these inflammatory markers are not sufficient in the detection of sole periapical inflammation.
The results of this cross-sectional study revealed that not all odontogenic inflammatory processes are able to stimulate systemic inflammation. Only periodontitis and pulp necrosis are important sources of systemic microinflammation in CKD patients. Plasma concentrations of IL-6 and TNF-α appear to be better, than CRP level, markers of odontogenic inflammation in CKD patients.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The project was supported by grant from Polish Committee for Scientific Research (Grant No. 40602431/0777).
References
- Thorman R, Neovius M, Hylander B. Clinical findings in oral health during progression of chronic kidney disease to end-stage renal disease in a Swedish population. Scand J Urol Nephrol. 2009;43:154–159
- Kshirsagar AV, Moss KL, Elter JR, Beck JD, Offenbacher S, Falk RJ. Periodontal disease is associated with renal insufficiency in the Atherosclerosis Risk In Communities (ARIC) study. Am J Kidney Dis. 2005;45:650–657
- Shultis WA, Weil EJ, Looker HC, et al. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30:306–311
- Arbes SJ, Slade GD, Beck JD. Association between extent of periodontal attachment loss and self-reported history of heart attack: an analysis of NHANES III data. J Dent Res. 1999;78:1777–1782
- Barkhordar RA, Hussain MZ, Hayashi C. Detection of interleukin-1 beta in human periapical lesions. Oral Surg Oral Med Oral Pathol. 1992;73:334–336
- Barkhordar RA, Hayashi C, Hussain MZ. Detection of interleukin-6 in human dental pulp and periapical lesions. Endod Dent Traumatol. 1999;15:26–27
- Danin J, Linder L, Lundqvist GR, Wretlind B. Cytokines in periradicular lesions: the effect of linezolid treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:492–498
- Franek E, Blaschyk R, Kolonko A, et al. Chronic periodontitis in hemodialysis patients with chronic kidney disease is associated with elevated serum C-reactive protein concentration and greater intima-media thickness of the carotid artery. J Nephrol. 2006;19:346–351
- Mattila K, Vesanen M, Valtonen V, et al. Effect of treating periodontitis on C-reactive protein levels: a pilot study. BMC Infect Dis. 2002;2:30–32
- D'Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156–160
- Silness J, Löe H. Periodontal disease in pregnancy. 3. Response to local treatment. Acta Odontol Scand. 1966;24:747–759
- Russell AL. The periodontal index. J Periodontol. 1967;38 (Suppl):585–591
- Landray MJ, Wheeler DC, Lip GY, et al. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the Chronic Renal Impairment in Birmingham (CRIB) Study. Am J Kidney Dis. 2004;43:244–253
- Chen LP, Chiang CK, Chan CP, Hung KY, Huang CS. Does periodontitis reflect inflammation and malnutrition status in hemodialysis patients. Am J Kidney Dis. 2006;47:815–822
- Kshirsagar AV, Elter JR, Craig R, Beck JD, Offenbacher S, Falk RJ. Periodontal disease is associated with renal insufficiency in III NHANES. Long-Term Care Interface. 2005;6:23–25
- Hujoel PP, White BA, Garcia RI, Listgarten MA. The dentogingival epithelial surface revised. J Periodontol Res. 2001;36:48–55
- Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PME, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71:1528–1534
- Noack B, Gencko RJ, Trevisan M, Grossi S, Zambon JJ, de Nardin E. Periodontal infection contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221–1227
- Ebersole JL, Machen RL, Steffen MJ, Willmann DE. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin Exp Immunol. 1997;107:347–352
- Fredriksson M, Figueredo C, Gustafsson A, Bergstrom K, Asman B. Effect of periodontitis and smoking on blood leukocytes and acute-phase proteins. J Periodontol. 1999;70(11):1355–1360
- Slade GD, Offenbacher S, Beck JD, Heiss G, Pankow JS. Acute-phase inflammatory response to periodontal disease in the U.S. population. J Dent Res. 2000;79(1):49–57
- Nakajima T, Honda T, Domon H, et al. Periodontitis-associated up-regulation of systemic inflammatory mediator level may increase the risk of coronary heart disease. J Periodont Res. 2010;45:116–122
- Mengel R, Bacher M, Flores-De-Jacoby L. Interactions between stress, interleukin-1b, interleukin-6 and cortisol in periodontally diseased patients. J Clin Periodontal Res. 2002;29:1012–1022
- Buhlin K, Gustafsson A, Pockley AG, Frostegard J, Klinge B. Risk factors for cardiovascular disease in patients with periodontitis. Eur Heart J. 2003;24:2099–2107
- Ikezawa I, Tai H, Shimada Y, Komatsu Y, Galicia JC, Yoshie H. Imbalance between soluble tumour necrosis factors receptor type 1 and 2 in chronic periodontitis. J Clin Periodontol. 2005;32:1047–1054
- Gorska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madalinski K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodnotol. 2003;30:1046–1052
- Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35:277–290
- Nadeem M, Stephen L, Schubert C, Davids MR. Association between periodontitis and systemic inflammation in patients with end-stage renal disease. SADJ. 2009;64:470–473
- Rahmati MA, Craig RG, Homel P, Kaysen GA, Levin NW. Serum markers of periodontal disease status and inflammation in hemodialysis patients. Am J Kidney Dis. 2002;40:983–989
- Pussinen PJ, Jousilahti P, Alfthan G, Palouso T, Asikainen S, Salomaa V. Antibody to periodontal pathogens are associated with coronary heart disease. Aterioscler Thromb Vasc Biol. 2003;23:1250–1254
- Marcaccini AM, Meschiari CA, Sorgi CA, et al. Circulating interleukin-6 and high-sensitivity C-reactive protein decrease after periodontal therapy in otherwise healthy subjects. J Periodontol. 2009;80:594–602
- Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM, Offenbacher S. Relationship of periodontal disease to carotid artery intima-media wall thickness: the Atherosclerosis Risk in Communities (ARIC) Study. Aterioscler Thromb Vasc Biol. 2001;21:1816–1822
- Neville BW, Damm DD, Allen CM, Bouquort JR. Oral and Maxillofacial Pathology. Philadelphia, PA: W. B. Saunders; 1995
- Prso IB, Kocjan W, Simic H, et al. Tumor necrosis factor-alpha and interleukin 6 in human periapical lesions. Mediators Inflamm. 2007;2007:38210
- Danin J, Linder LE, Lundqvist G, Andersson L. Tumor necrosis factor-alpha and transforming growth factor beta1 in chronic periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:514–517

